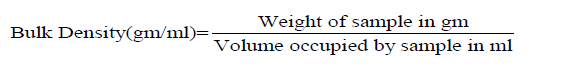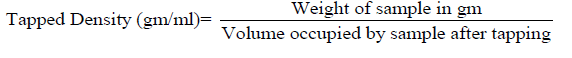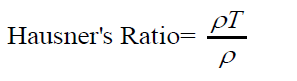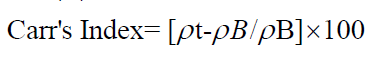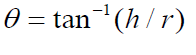Keywords
Nifedine; Extended release tablet; Infrared Spectroscopy; Push pull layer
Introduction
Oral route is the most oldest and convenient route for the administration of therapeutic agents because of low cost of therapy and ease of administration leads to higher level of patient compliance. Approximately 50% of the drug delivery systems available in the market are oral drug delivery systems and historically too, oral drug administration has been the predominant route for drug delivery. It does not pose the sterility problem and minimal risk of damage at the site of administration. During the past three decades, numerous oral delivery systems have been developed to act as drug reservoirs from which the active substance can be released over a defined period of time at a predetermined and controlled rate. The oral controlled release formulation have been developed for those drug that are easily absorbed from the gastrointestinal tract (GIT) and have a short half-life are eliminated quickly from the blood circulation. As these will release the drug slowly into the GIT and maintain a constant drug concentration in the plasma for a longer period of time The sustained release [1], sustained action, prolonged action, controlled release, extended action, timed release, depot and respiratory dosage forms are terms used to identify drug delivery system that are designed to achieve a prolonged therapeutic effect by continuously releasing medication over an extended period of time after administration of a single dose. Extended release formulation is an important program for new drug research and development to meet several unmet clinical needs [2]. There are several reasons for attractiveness of these dosage forms viz. provides increase bioavailability of drug product, reduction in the frequency of administration to prolong duration of effective blood levels, Reduces the fluctuation of peak trough concentration and side effects and possibly improves the specific distribution of the drug [3].
Extended release drug delivery system achieves a slow release of the drug over an extended period of time or the drug is absorbed over a longer period of time [4]. Extended release dosage form initially releases an adequate amount of drug to bring about the necessary blood concentration (loading dose, DL) for the desired therapeutic response and therefore, further amount of drug is released at a controlled rate (maintenance dose, DM) to maintain the said blood levels for some desirable period of time [5]. The drugs that have to be formulated as an ERDDS should meet following parameters. It should be orally effective and stable in GIT medium [6,7]. Drugs that have short half-life, ideally drug with a half-life in the range of 2-4 hrs. Makes a good candidate for formulation into ER dosage forms e.g. Captopril, Salbutamol sulphate. The dose of the drug should be less than 0.5 g as the oral route is suitable for drugs given in dose as high as 1.0 g e.g. Metronidazole [8]. Therapeutic range of the drug must be high [9]. A drug for ERDDS should have therapeutic range wide enough such that variations in the release do not result in concentration beyond the minimum toxic levels [10].
Materials and Methods
Nifedipine was obtained as gift sample Zydus Cadila, Mumbai, India. Polyethylene oxide and HPMC was obtained from Lucid Group, Mumbai, polyvinyl pyrrolidone was obtained from SD Fine chem, Mumbai. All other ingredients used were of analytical grade
Screening of excipients
Drug entraining/suspending polymers: There are limited number of excipients which exhibit requirements for hydration and viscosity in the drug layer of the tablet core. Water soluble polymers such as polyethylene oxide, hydroxypropyl methylcellulose (HPMC) and poly Vinyl Pyrrolidone (PVP) have been used for entrainment of drug. However from literature survey it is found that polyethylene oxide is widely used for entraining of drug. Polyethylene oxide is available in wide range of molecular weight but average molecular weight 100,000 to 400,000 is most commonly used for purpose of drug entraining. The exclusive use of Polyox is due to its ability to wet and hydrate to appropriate viscosity range to suspend a drug particle and flow through the delivery port. Also PEO based drug layer work well with hydration and swelling of PEO based swelling layer.
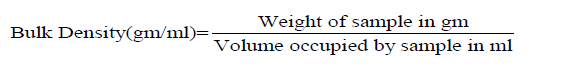
Tapped density: Tapped density is defined as the ratio of mass of a powder to the tapped volume calculated using tap density meter.
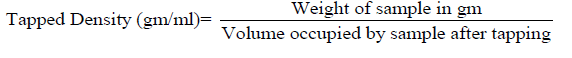
Hausner’s ratio: The Hausner’s ratio is a number which indirectly measure the flow ability of a powder or granular material. The Hausner’s ratio is calculated by the formula.
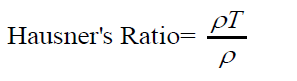
Where, ρT; is the freely settled bulk density of the powder, and ρT is the tapped bulk density of the powder.
Carr’s index: Carr’s index gives the measure of compressibility of powder. The Carr’s Index measures the propensity of powder to be compressed. The packing ability of drug was evaluated from change in volume, which is due to rearrangement of packing occurring during tapping. It is indicated as Carr‘s compressibility index (CI) and can be calculated as follows:
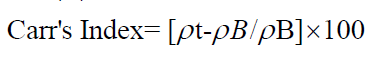
Where, ρβ is the freely settled bulk density of the powder, and ρT is the tapped bulk density of the powder.
Angle of repose: Angles of repose have been used as indirect methods of quantifying powder flow ability, because of their relationship with interparticle cohesion. Angle of repose (θ) of powder of both drug layer and push layer was determined by funnel method. The powder of both drug layer and push layer was allowed to flow through funnel freely onto the clean surface. Funnel was placed in such a height that bottom tip of funnel should not touch the apex of the heap of granules. Height and radius of cone were measured through scale and values were placed in equation.
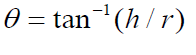
Where, θ = angle of repose; h = height of cone; r = radius of cone base.
Results and Discussion
Standardization of Nifedipine
Description: When drug was tested for its organoleptic properties it was observed that, drug as white, odourless, bitter in taste and microcrystalline in nature, freely soluble in water, in methanol and in methylene chloride, slightly soluble in ethanol.
Spectroscopic analysis
UV Spectroscopy: The scan of a 10 μg/mL solution of the Nifedipine in deionized water is as shown in Figure 1. The Nifedipine was found to have absorption maxima was nm having molar absorptivity 0. 543. Figure 1 gives the UV Spectrum of Nifedipine (10 μg/mL) analyzed on Jasco-V-530 UV spectrophotometer in Deionized water. Fourier Transmission Infrared Spectroscopy.
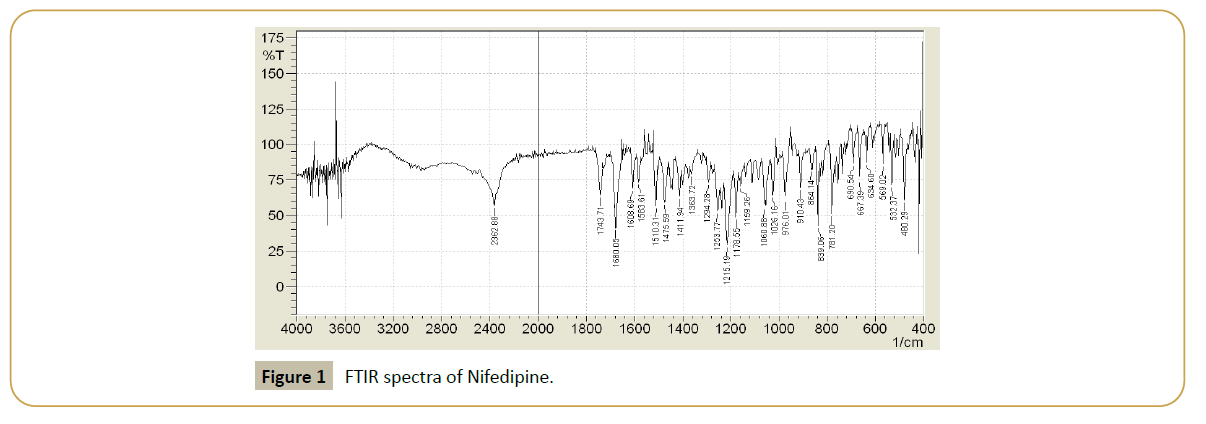
Figure 1: FTIR spectra of Nifedipine.
The IR spectrum of the drug, Nifedipine which was recorded on Jasco FTIR
5300 spectrophotometer by KBr disk method is as shown in Figure 1. The spectra shows characteristic peaks of functional group of Nifedipine as shown in Table 1.
| Functional group |
Reported peak |
Observed peak |
| N-Hstretch |
2393 |
2362 |
| C=Ostretch for acetate |
1743 |
1743 |
| C=Ostretch for cyclic ketone |
1679 |
1680 |
| C-Hbending |
900-650 |
900-650 |
| P-substituted aromatic C-H out of plane deformation |
781 |
781 |
| O-substituted aromatic C-H out of plane deformation |
839 |
839.06 |
Table 1: Characteristic IR peak of functional group
Differential Scanning Calorimetry (DSC)
The DSC endotherm of Nifedipine was characterized by single sharp melting endotherm at 219.5ºC which was found to be in agreement to the reported melting endotherm (Figures 2-9, Tables 2 and 3)
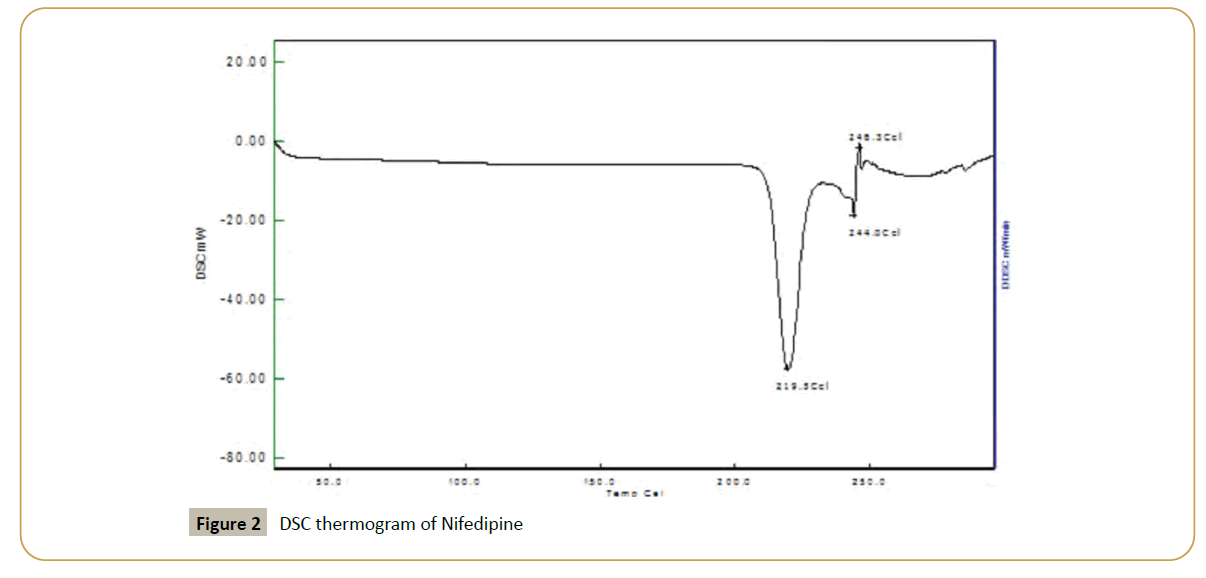
Figure 2: DSC thermogram of Nifedipine
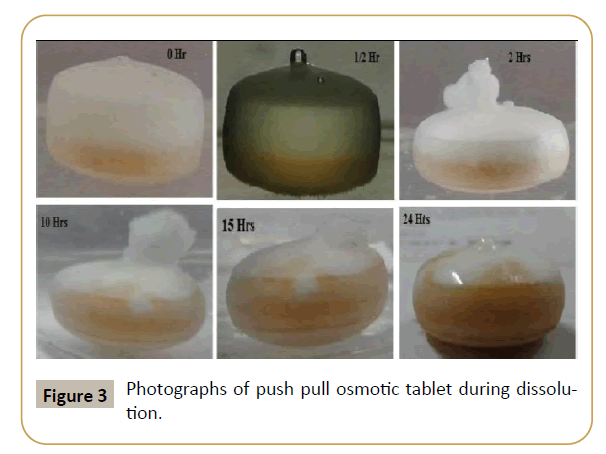
Figure 3: Photographs of push pull osmotic tablet during dissolution.
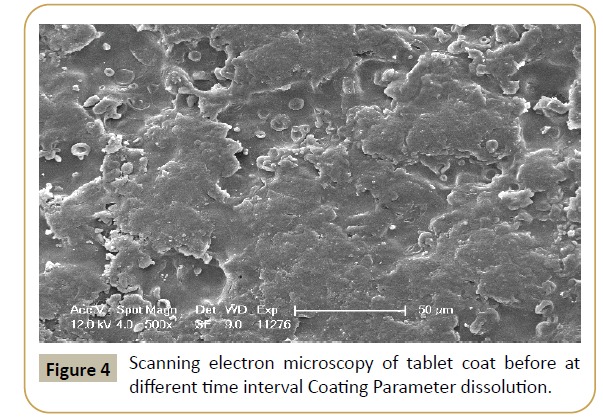
Figure 4: Scanning electron microscopy of tablet coat before at different time interval Coating Parameter dissolution.
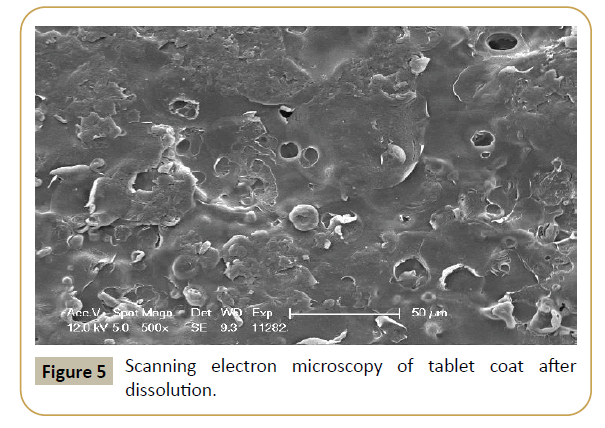
Figure 5: Scanning electron microscopy of tablet coat after dissolution.
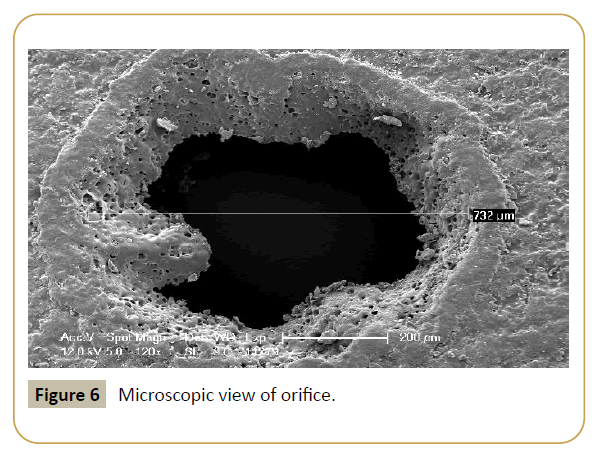
Figure 6: Microscopic view of orifice.
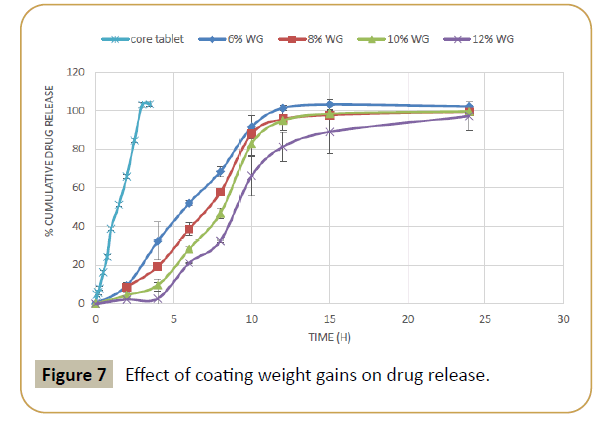
Figure 7: Effect of coating weight gains on drug release.
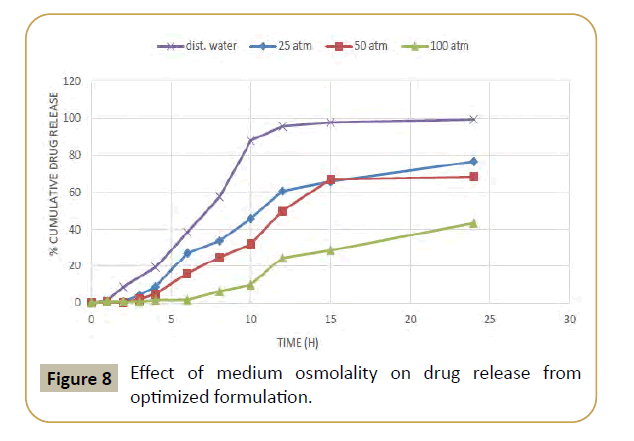
Figure 8: Effect of medium osmolality on drug release from optimized formulation.
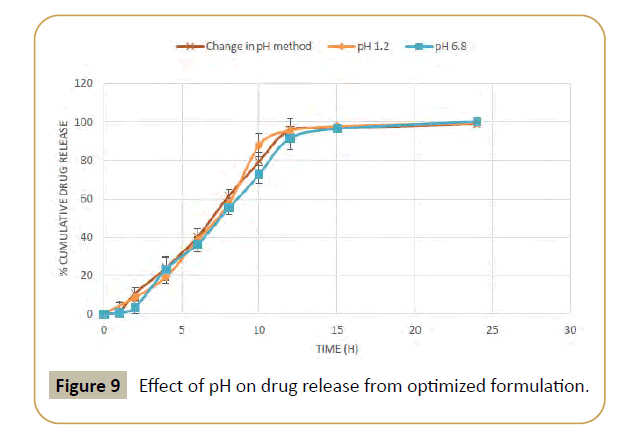
Figure 9: Effect of pH on drug release from optimized formulation.
| Sr. no |
Ingredients |
mg/tab |
Scale up batch-II 150000 Tab(Kg) |
| 1 |
Nifedipine USP+IH* |
99** |
14.85 |
| 2 |
Polyethylene Oxide NF (POLYOX WSR N N 80) |
355.405 |
53.311 |
| 3 |
Hypromellose USPType 2910 (Methocel Premium E5 LV) |
24.75 |
3.713 |
| 4 |
Potassium Chloride USP |
9.9 |
1.485 |
| 5 |
Colloidal Silicon Dioxide NF |
2.475 |
0.371 |
| 6 |
Magnesium Stearate NF |
3.47 |
0.52 |
| 7 |
Isopropyl Alcohol USP |
-- |
5.31 |
| 8 |
Methyl Alcohol NF |
-- |
5.31 |
| |
Average weight for Drug layer |
495 |
74.25 |
| |
PUSH LAYER |
|
180000 Tab $ |
| 9 |
Polyethylene Oxide NF (POLYOX WSR Coagulant) |
170.283 |
30.651 |
| 10 |
Sodium Chloride USP |
72.6 |
13.068 |
| 11 |
Ferric Oxide red NF |
1.65 |
0.297 |
| 12 |
Isopropyl Alcohol USP |
-- |
1.293 |
| 13 |
Methyl Alcohol NF |
-- |
0.144 |
| 14 |
Colloidal Silicon Dioxide NF |
1.237 |
0.223 |
| 15 |
Magnesium Stearate NF |
1.73 |
0.311 |
| |
Average weight for push layer |
247.5 |
44.55 |
| |
Average weight of uncoated bi-layer tablet |
742.5mg |
|
| 16 |
Cellulose Acetate NF 398/10 |
42.75 |
7.695# |
| 17 |
Polyethylene Glycol NF 4000 |
2.25 |
0.405# |
| 18 |
Methylene Chloride NF |
-- |
124.56# |
| 19 |
Methyl Alcohol NF |
-- |
20.63# |
| 20 |
Opadry Brown 03B86636 IH |
44 |
7.92# |
| |
Total average weight of Coated Tablet |
831.5mg |
|
Table 2: Optimized formula of push-pull osmotic tablet of Nifedipine
| Time in hour |
% Cumulative drug release (Mean ± S.D)(N=3) |
USP discussion test 6 limits |
| 0 |
0 |
0 |
| 1 |
1.3981±1.2 |
-- |
| 2 |
10.534±2.4 |
Not more than 25% |
| 4 |
23.853±3.4 |
25-50% |
| 6 |
40.288±4.1 |
-- |
| 8 |
61.539±2.5 |
60-85% |
| 10 |
79.510±2.2 |
-- |
| 12 |
95.910±3.3 |
Not less than 70% |
| 15 |
97.691±4.2 |
Not less than 80% |
| 24 |
99.169±1.1 |
-- |
Table 3: Cumulative percent release of Nifedipine with respective to time
The drugs used for chronic diseases require close monitoring to avoid unwanted side effects caused by fluctuation in plasma drug concentration. Immediate release dosage forms are associated with certain problems such as fluctuation of plasma drug concentration which leads to under or over medication and may cause adverse effects in case of drugs with narrow therapeutic index and lead to patient noncompliance due to repeated dose administration. The constant intravenous infusion is one of the promising delivery system which maintains a constant blood concentration of drug but it is not feasible for long term treatment and it also compromises the patient compliance.
Hence to avoid plasma fluctuation of drug concentration by frequent administration of immediate dosage forms and also to avoid patient noncompliance due to invasive therapy, the oral controlled release drug delivery is a promising drug delivery system. Which release the drug in a predetermined, predictable, and reproducible manner? Despite many advantages of oral controlled release dosage forms, they may be affected by pH, gastric motility, ionic strength and presence of food. One of the promising drug delivery system is osmotic drug delivery system which overcomes such side effects and releases the drug at zero order. The drug release is predetermined, predictable, and in a reproducible manner which is independent of pH, gastric motility, ionic strength and presence of food.
Conclusion
Nifedipine is a calcium channel blocker widely used for the treatment of hypertension, arrhythmia and for angina pectoris caused by coronary arterial spasm. The drug undergoes rapid elimination which causes a short half-life (2-4 hrs), frequent dosing of three times per day. Therefore, nifedipine, with its low oral bioavailability, short half-life, and multiple daily dosing, is appropriate candidate for a formulation in an extended-release, once-a day dosage form. The main objective of the present study was to design and development of nifedipine extended release tablets 90 mg with zero order rate of drug delivery for a desired period of time. Nifedipine extended release tablet 90 mg utilizes a unique osmotic drug delivery system. This formulation allows for single daily dose, because of the ability to deliver a constant and uniform dose of Nifedipine, it is therefore characterized as a zero order kinetic system. Another unique feature of this system is that each tablet is formulated with 10% more drug than tablet strength indicates. This excesses quantity of drug is necessary because approximately 10% of the drug remains within the tablet and cannot be expelled by the expanding osmotic push layer. The intact tablet and remaining drug is then expelled in the feces. Other important characteristic of this system is its ability to maintain a unique release rate of Nifedipine despite fluctuation in environmental condition. This formulation maintains a consistent rate of release at physiological pH range of 1.2-7.4. In addition the degree of gastrointestinal motility doesn’t affect the drug release rate.
Acknowledgement
The authors are thankful to the Management, Nootan College of Pharmacy for providing necessary facilities to carry out this work.
32723
References
- Rajajayarao Y, Divya P, Divyasree K, Manohar Babu (2014) Formulation and Evaluation of Matrix type sustained release Nifedpine tablets. IJRPC 4:34-45.
- Amol Choudhary (2011) Formulation and Development of Extended released Tablets of lamotrignine,. Int J Pharm Bio Sci 2:198-210.
- Sunil K, Kumar A, Gupta V (2012) Oral extended Drug Delivery System promising approach. Asian J Res Pharm Sci 2: 101-106.
- Jayathi B, Manna PK (2011) Per oral Extended Release Products-Review. J App Pharm Sci 1: 50-55.
- Prabakaran L, Vishalini M (2010) Hydrophilic Polymer matrix systems of Nifedipine sustained release tablets:formulation optimization by prsponce surface method. Der pharmacia sinica. 1:147-165.
- Soumik G (2010) Formulation and Evaluation of Sustained Release Dosage Form of Nifedipine Hydrochloride using multiunit chitosan treted alginate. Int J Pharm Biomed Res 1:124-131.
- Jayachandar G, Johannes K, Vinod PS (2015) Biowaiver Monographs for Immediate-Release Solid Oral Dosage Forms: Nifedipine. J Pharm Sci 104:3289-3298.
- Christina K (2016) Dissolution Enhancement of the poorly soluble drug Nifedipine by co-spry drying with microporous zeolite beta. J Drug Deli Sci Tech 35: 91-97.
- Martin K, Rene H, David P E (2016) Methodology of oral formulation selection in the pharmaceutical industry. Eur J Pharm Sci 87:136-163.
- Morteza S, Amirali E, Timothy L (2016) A novel formulation for solubility and content uniformity enhancement of poorly water-soluble drugs using highly-porous mannitol. Eur J Pharm Sci 83:52-61.