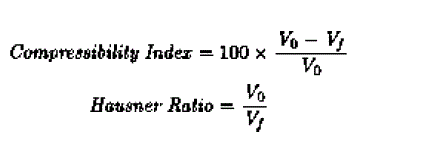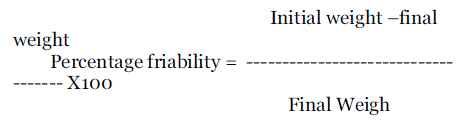Key words
|
| |
| Aceclofenac; topical gel; carbopol; hydroxypropylmethylcellulose; carboxymethylcellulose; sodium alginate; penetration enhancer; drug release |
| |
Introduction
|
| |
| During the last decade many investigations have been carried out with the aim of discovering an ideal formulation for colon-specific drug delivery. Many approaches have been demonstrated [1]. The colon is a site where both local and systemic drug delivery can take place. Treatment might be more effective, if the drug substances were targeted directly on the site of action in the colon. Lower doses might be adequate and, if so, systemic side effects might be reduced [2, 3]. The gastrointestinal tract presents several formidable barriers to the drug delivery. Colonic drug delivery has gained increased importance not just for the delivery of drugs for the treatment of local diseases of colon but also for its potential for the delivery of proteins and peptides. The colon presents less hostile conditions for drug delivery because of less diversity and intensity of enzymatic activities and a near neutral pH [4, 5]. |
| |
| In addition, the colon may be the best site for drug delivery because of the long residence time and the low digestive enzymatic activities this may be useful for prolonged drug delivery [6, 7]. |
| |
| Colon drug delivery is a relatively recent approach to the treatment of diseases and irritable bowel syndrome, recommended treatments include the administration of anti-inflammatory drugs, chemotherapy and antibiotics, which must be released in colon. Such local treatment has the advantages of requiring small drug quantities, possibly leading to reduce the incidence of side effects and drug interactions [8]. The usual treatment of anti-inflammatory bowel disease consists of frequent intake of anti-inflammatory drugs at high doses in order to induce remission of active diseases leads to side effects like dizziness, GI disturbances, head ache and skin rash [9]. |
| |
| The functional requirement of an oral colonic drug delivery system is twofold a robustness of form to prevent drug release in the upper gastrointestinal regions and sensitivity to the trigger mechanism to ensure prompt drug release in the colon. The pH dependent approach for colonic drug delivery is based on the pH differential along the gastrointestinal tract with values increasing from about 1 to 2.5 in the stomach through 6.6 in the proximal small bowel to a peak of about 7.5 in the terminal ileum followed by a fall in pH to 6.4 in the colon [10, 11]. This concept utilizes polymeric carriers that are insoluble in the low pH media of the upper gastrointestinal tract, but dissolve at the higher, near neutral pH of the distal gut [12, 13]. The current perspective of this study is to prolong the release of the anti- inflammatory drug mesalamine to the colon using one such pH dependent polymeric systems, this new system will stay intact and enable the drug to be delivered in a delayed manner in order to provide effective treatment for IBS. |
| |
| This system is designed in such a way to provide complete protection over the entire intestinal section including the small intestine, the three coats which encloses a acid soluble layer with Eudragit E and a middle inert layer using HPMC and an outer most layer Eudragit S the drug is aimed at releasing most of the drug to the colonic part which is shown in the Figure 1. |
| |
MATERIALS
|
| |
| Mesalamine obtained as gift sample from Micro lab, Bangalore. Micro crystalline cellulose( MCC), Lactose, Citric acid and Poly vinyl pyrolidone (PVP), Isopropyl alcohol (IPA), Sodium starch glycolate (SSG), Magnesium stearte (MS), Aerosil |
| |
METHODS
|
| |
|
Drug and the polymer compatibility study
|
| |
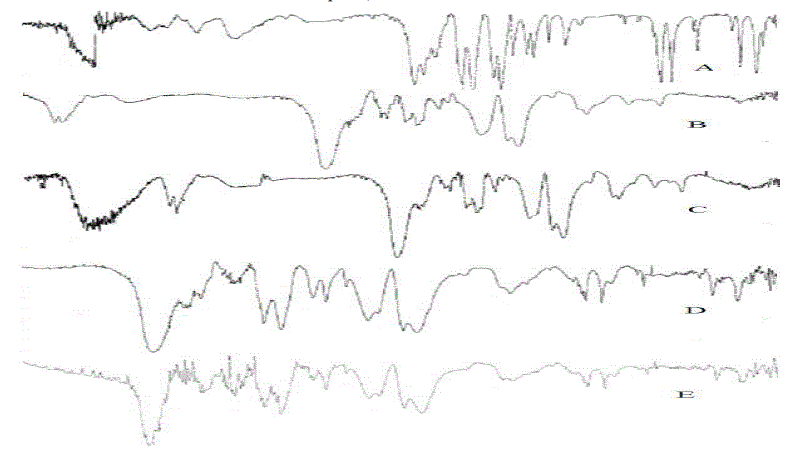
| FT-IR Study over the drug and the polymers were performed and then analyzed for specific interactions. |
| |
|
Particle size analysis for the raw drug [9,14]
|
| |
| The principles of electronic and light sensing and light scattering techniques have been used to develop automated particle size counters that indirectly measure particle size. |
| |
| Accurate measurement of particle size is essential for the determination of the consistency, quality, and overall usefulness and performance characteristics of a material. The instrument selected for a particle sizing application must be appropriate for the material to be measured and for the environment in which the instrument is to be used. |
| |
| Laser diffraction or low angle laser light scattering has become one of the preferred methods for particle size characterization. The instrument consists of a laser light source (generally a He-Ne gas laser), a suitable detector such as silicon photodiode, and a means of passing the sample through the laser beam. An ultrasonic probe may be used to improve particle dispersion. In this technique, particles are dispersed in a liquid or gaseous medium. The diffraction angle is inversely proportional to particle size. |
| |
| The instrument does not require a calibration against a standard and the method is non destructive and samples can be recovered after testing. The latest instrument utilizes the Mie theory of particle interaction with light and allow for accurate measurements over a large size range (0.1 to 3000μm). |
| |
|
Procedure
|
| |
| The particle size analyzer used is Malvern particlesizer MS 2000, mesalamine and the granules of successful batches were analyzed by using the particlesizer. Dry analysis was performed over the mesalamine raw material and the granules batches using Scirocco 2000 accessory, place 200gms of sample in general purpose tray and close the lid then the manual measurement is started with a 2.5 bar pressure, the software measures the background automatically and then after entering the respective documentation for the mesalamine such as refractive index, absorption index, sample name, source type, source name, bulk/lot ref no and after observing the obscuration value the measurement tab is clicked to measure the particle size of the material. The analysis was done in triplicates to get the mean value of the particles size present in the provided sample |
| |
|
Physical evaluation of mesalamine raw drug [9]
|
| |
| Physical evaluation over the mesalamine raw drug was performed such as angle of repose, compressibility index, Hausner’s ratio, and bulk density characteristics. |
| |
|
Formulation of mesalamine tablets by wet granulation
|
| |
| Core tablets of mesalamine USP were prepared by wet granulation technique using PVP as a binder. Lactose was used as a diluent and was granulated by passing through sieve no 10 and prior to granulation by passing all the materials were passed through 10mesh. |
| |
| The dried granules were passed through mesh no 20 and these granules were lubricated with the mixture of aerosil and magnesium stearate. Finally granules were compressed into tablets using rotary tablet press (M/S RIMEK). The prepared tablets of each batch were subjected for evaluation of hardness test, friability, drug content and in vitro drug release studies. |
| |
|
Physical evaluation of granules [9]
|
| |
| The wide spread use of powders in the pharmaceutical industry has generated a variety of methods for characterizing the flow. The development of such a variety of test methods was inevitable powder behavior is multifaceted and thus complicates the effort to characterize powder flow. |
| |
|
Angle of repose [15, 16]
|
| |
| Angle of repose is a characteristic related to interparticulate friction or resistance to movement between particles. Angle of repose test results are reported to be very dependent upon the method used. Experimental difficulties arise due to segregation of material and consolidation or aeration of the powders as the cone is formed. |
| |
| The angle of repose is a constant three-dimensional angle (relative to the horizontal base) assumed by a cone like pile of materials formed. The granules were allowed to fall freely through a funnel fixed at 1cm above the horizontal flat surface until apex of conical pile just touches the tip of the funnel. |
| |
| The angle of repose was determined by the formula |
| |
| θ = Tan-1h/r |
| |
| h = Height of pile |
| |
| r = Radius of the pile formed by the granules on the ground. |
| |
|
Compressibility index and Hausners ratio [15,16]
|
| |
| These are measures of the propensity of a powder to be compressed. As such they are measures of the relative importance of interparticulate interactions. In a free flowing powder such interactions are generally less significant and the bulk and tapped densities will be closer in value for poorer flowing materials, there are frequently greater differences between the bulk and tapped densities will be observed. |
| |
| The compressibility index has been proposed as an indirect measure of bulk density, size and shape, surface area, moisture content and cohesiveness of materials. |
| |
| The compressibility index and the hausner ratio are determined by measuring both the unsettled apparent volume (Vo) and final tapped volume (Vf) of the powder. The compressibility index and the hausner ratio are calculated as follows |
| |
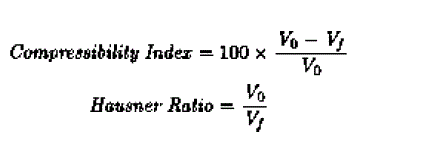 |
| |
| Weigh 5gms of dried granules which were previously passed through 20mesh screen and taken in a 25ml measuring cylinder and transfer the weighed granules in the measuring cylinder and find out the apparent volume and after 100 tappings measure the final volume repeat the procedure for three consecutive times and record the result. |
| |
|
Particle size analysis of the granules
|
| |
| Particle size analysis of the granules F 01(core1), F 02 (core2) were analyzed using Malvern particle size analyser MASTERSIZER 2000 |
| |
|
Procedure
|
| |
| The particle size analyzer used is Malvern particlesizer MS 2000, mesalamine and the granules of successful batches were analyzed by using the particlesizer. Dry analysis was performed over the mesalamine raw material and the granules batches using Scirocco 2000 accessory, place 200gms of sample in general purpose tray and close the lid then the manual measurement is started with a 2.5 bar pressure, the software measures the background automatically and then after entering the respective documentation for the mesalamine such as refractive index, absorption index, sample name, source type, source name, bulk/lot ref no and after observing the obscuration value the measurement tab is clicked to measure the particle size of the material. The analysis was done in triplicates to get the mean value of the particles size present in the provided sample. |
| |
|
Evaluation of tablets
|
| |
|
Physicochemical evaluation of formulated tablets [17, 18]
|
| |
|
Hardness test
|
| |
| The resistance of the tablets to chipping, abrasion or breakage under the condition of storage, transportation and handling before usage depends on its hardness. Several devices are used to test tablet hardness, Monsanto tester, Strong-cobb tester, Pfizer tester, Erweka tester and the Schlleuniger tester. |
| |
| Unit for hardness is Kg/cm2. The optimum hardness regarded for uncoated tablet is 4-6Kg/cm2. |
| |
|
Friability test
|
| |
| It’s a measure of mechanical strength of tablet Using Roache apparatus performed the test. The pre weighed tablets were placed in the friabilator, friabilator consist of a plastic chamber that revolves at 25rpm, chopping the tablets at a distance of 6inches in each revolution the tablets were related in the friabilator for 4min. At the end of test, tablets were dusted and reweighed. The loss is weighed of tablet is the measure of friability and is expressed in percentage as |
| |
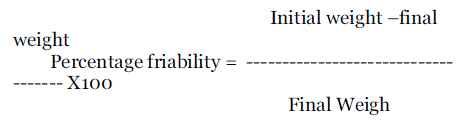 |
| |
| Conventional compressed tablets that lost less than 0.5 -1% of their weight were generally considered acceptable |
| |
|
Uniformity of weight
|
| |
| A tablet designed to contain a specific amount of the drug in a specific amount of tablet formula, weight of the tablet being made is routinely measured to ensure that a tablet contains the proper amount of the drug. USP weight variation test was run by weighing 20 tablets individually calculated average weight and compared the individual tablet weights to the average weight. |
| |
| The tablet meets the USP test if not more than 2 tablets are outside the percentage limit and if no tablet differs by more than 2 times the percentage limit. |
| |
|
Disintegration Test
|
| |
| For most of the tablets, first important step towards the solution is the break down of the tablet in to smaller particle or granules, a process known as disintegration. The USP device used to test disintegration contains six glass tubes that are 3 inches long, open at the top and held against a 10- mesh screen at the bottom end of the basket rack assembly. |
| |
| To test disintegration, Place one tablet in each of the six tubes of the basket rack was positioned in a one liter beaker of water, simulated gastrointestinal fluid or simulated intestinal fluid maintained at 37±2oc such that tablets remain 2.5cm below the surface of the liquid on their upward movement and descend not closer than 2.5cm from the bottom of the beaker. A standard motor drive device was used to move the basket assembly containing the tablets up and down through the distance of 5-6cm at a frequency of 28to32 cycles/min. As the immersion fluid after one hour of operation in simulated gastrointestinal fluid lift the basket form the fluid and observe the tablets; the tablets show no evidence of disintegration, cracking or softening. Operate the apparatus using simulated intestinal fluid maintained at 37±2oc as the immersion fluid for the time specified. |
| |
|
Determination of drug content
|
| |
| Diluent: Water |
| |
| Standard Preparation: Weigh accurately 25.0 mg of each tablet should be between 90 to 110% of the Mesalamine in 25 ml volumetric flask and add 5 ml of 0.25N stated labeled amount. |
| |
| Hydrochloric Acid, swirl to dissolve and make upto the mark with Milli-Q water. Pipette out 2 ml from the above solution in to 50 ml volumetric Flask and Make upto mark with water. (Conc. 40mcg/ ml) |
| |
| Sample Preparation: Place one tablet in a 50 ml volumetric flask and add 5 ml of 0.25N Hydrochloric Acid swrill and sonicate for 10 minutes (or until dissolve) then make upto volume with water. Pipette out 2 ml of the above solution in to 50 ml volumetric flask and made upto volume with water. (Conc. 60mcg/ ml) |
| |
| Methodology: Inject 3 μl of the standard (replicate) and sample preparation (duplicate) and record the chromatogram. |
| |
| Acceptance Criteria: The content of Mesalamine in |
| |
|
Coating of core tablets
|
| |
| The prepared core tablets were coated with solutions of Eudragit, the percentage increase in weight was measured as 5%, 7.5%, and 10%, both the batches were coated with similar percentages of coating materials and isopropyl alcohol and methylene chloride were used as solvents in the ratio of 40:60. Polyethylene glycol was used as plasticizer at a concentration of 10% |
| |
| Our formulations with both the batches were coated with the triple layer coating apart form conventional coating it has three coatings. It has Eudragit E100 as an outer coating next to core and HPMC as a middle inert layer and Eudragit S100 as an outer most enteric coating to protect the tablet form intestinal contents. Percentage increase in weight was measured as 8%, 2%, and 6% respectively. |
| |
|
Optimization of coating formula for Eudragit
|
| |
| In the similar manner coating solution for Eudragit E100 and HPMC the inert layer was prepared and then it was applied to the core tablets over the E100 layer. Eudragit S100 was coated to the F01 and F02 batches. The percentage increase in weight was measured as 8%, 2%, and 6% respectively. |
| |
|
In vitro evaluation of enteric coated tablets
|
| |
| Disintegration test |
| |
| For most of the tablets, first important step towards the solution is the break down of the tablet in to smaller particle or granules, a process known as disintegration. The USP device used to test disintegration contains six glass tubes that are 3 inches long, open at the top and held against a 10- mesh screen at the bottom end of the basket rack assembly |
| |
| To test disintegration, Place one tablet in each of the six tubes of the basket rack was positioned in a one liter beaker of water, simulated gastrointestinal fluid or simulated intestinal fluid maintained at 37±2°c such that tablets remain 2.5cm below the surface of the liquid on their upward movement and descend not closer than 2.5cm from the bottom of the beaker. A standard motor drive device was used to move the basket assembly containing the tablets up and down through the distance of 5-6cm at a frequency of 28to32 cycles/min. As the immersion fluid after one hour of operation in simulated gastrointestinal fluid lift the basket form the fluid and observe the tablets; the tablets show no evidence of disintegration, cracking or softening. Operate the apparatus using simulated intestinal fluid maintained at 37±2°c as the immersion fluid for the time specified [19, 20]. |
| |
|
Dissolution study [21, 22]
|
| |
| The ability of the prepared Mesalamine tablets to retard the drug release in the physiological environment of the stomach and the small intestine was assessed by conducting drug release studies in simulated stomach and small intestinal pH, respectively. The changing pH media, USP 29 type II, specific monograph for mesalamine-delayed release tablets were used. |
| |
|
Procedure
|
| |
|
Acid stage - pH 1.2:
|
| |
| Dissolution test was conducted in USP 29 type II apparatus at 100-rpm 37±0.5°C for 2hrs in 0.1M Hcl (500ml) as the average gastric emptying time is about 2h. 5ml of aliquot fluid was withdrawn, then the remaining solution was discarded and the tablets were retained in the proper order, so that each could be returned to its respective vessel later on. Blot the tablets with a paper towel to dry, and proceeded immediately as directed for buffer stage I in the specific monograph. |
| |
| Acceptance criteria- the drug release should be less than 1% |
| |
|
Buffer stage I - pH 6.0:
|
| |
| The dissolution medium was replaced with pH 6.0 phosphate buffer 900 ml tablets were placed in the respective vessels taken form acid stage with the equilibrated temperature of 37±0.5°C and tested for drug release for one hour with 100rpm. At the end of time period 5ml sample was taken and analyzed for mesalamine by UPLC method. After removing the sample to be analyzed, 50ml of aliquot was removed, and preceded immediately as directed for buffer stage two in the monograph. Acceptance criteria- The drug release should be less then 1% |
| |
|
Buffer stage II - pH 7.2
|
| |
| 50ml of sodium hydroxide solution was added to adjust the pH to 7.2 and continued the run for 90min and at the end an aliquot of sample was withdrawn and analyzed by using UPLC at 230nm. Acceptance criteria- the drug release should not be less than 80% in the phosphate buffer. |
| |
|
Stability studies [23, 24]
|
| |
| The term “Stability” with respect to a drug dosage form, refers to the chemical and physical integrity of the dosage unit, and refers to the chemical and physical integrity of the dosage unit, and when appropriate, the ability of the dosage unit to maintain protection against microbiological contamination. The stability parameters of a drug dosage form can be influenced by environmental conditions of storage (temperature, light, air and humidity), as well as the package components. |
| |
| Two batches of core tablets F01, F02 were subjected to stability studies and observed in regular intervals for physical changes for a time period of two months, at the end tablets were analyzed for the remaining drug content by UPLC method. The stability study was performed over the tablets of batches F01, F02, tablets which were subjected to RT( room temperature), CE (controlled environment 8°c) and VCE( variable environment 50°c), and the tablets were periodically checked for any significant changes. And after two months the tablets were analyzed for the remaining amount of drug present in the tablet. Both the batches were within the limit specified all the period of time. |
| |
RESULTS AND DISCUSSION
|
| |
|
Drug and the polymer compatibility study
|
| |
| FTIR analysis between the drug and enteric polymer mixture showed no unaccountable extra peaks, which confirms the absence of chemical interaction between the drug and polymer. |
| |
|
Particle size analysis for the raw drug
|
| |
| The particle size measurement report is given in the Table No 6. Mesalamine raw material was analyzed for physical properties such as particle size, bulk properties. The mean particle size of mesalamine was found as 15.499μm, the compressibility index was 47.019 and the bulk density was 0.226gm/m2 form the physical properties results were with in acceptable level except flow properties of the drug. |
| |
|
Physical evaluation of mesalamine raw drug
|
| |
| The data obtained indicates the poor flow nature of the mesalamine raw material, it may be due to the extreme moisture content in the API. This clearly explains that mesalamine should be subjected to granulation. |
| |
| Exploratory batches of mesalamine formulations ML01 to ML04 were prepared by wet granulation and evaluated for its physical properties of granules except ML01, which was formulated for direct compression. The result shows formulated granules ML01 to ML03 had poor flow behaviour, which were not satisfactory for the formulation of tablets. |
| |
| ML04 showed an angle of repose 27°4’, Hausner’s ratio 1.37 and compressibility index of 25%, which has a optimal flow characteristics. So ML04 was taken as a base for the core formulation of mesalamine. |
| |
| F01, F02 batches were prepared by obtained knowledge form the exploratory batches, which basically differed only in the amount of organic acid (retardant) added. F02 had a 10% of citric acid, where as F01 didn’t have any organic acid added into the formulation and the results showed F01 batches has an angle of repose of 24°6’, Hausner’s ratio 1.12 and compressibility index 11% and F02 batch showed 22°5’, 1.11, 9% of Angle of repose, Hausner’s ratio, compressibility index respectively. All to gather these two batches has a excellent flow characteristics. |
| |
|
Particle size analysis of the granules
|
| |
| The particle size measurement report is given in the Table No:6 |
| |
EVALUATION OF TABLETS (8)
|
| |
|
Physicochemical evaluation of formulated tablets
|
| |
|
Evaluation of coating formula
|
| |
| Eudragit S/E100 : Coating formula over core tablets were optimized by coating three batches of cores using three coating formulas. Among them Coat no:1 was vary sticky and the surface of the coated tablet looked ununiform and Coat no:3 was highly viscous and which was not able to coat. Coat no:2 had a uniform smooth coating over the cores. |
| |
| Coat no:2 was applied to both the formulations of F01, F02 with a percentage weight increase of 5%, 7.5%, 10% and triple coat was separately coated using Eudragit E100 as 8% coat over core and second layer comprising of coating layer HPMC as 2% over E100 and finally Enteric layer using Eudragit S100 of 6% weight increase. |
| |
|
In vitro evaluation of enteric coated tablets
|
| |
| Disintegration test |
| |
| Disintegration study of the coated tablets were performed both in simulated gastric fluid for 1 hour and then in simulated intestinal fluid for 2 hours. 5% coat immediately disintegrated <20min in simulated gastric fluid where as the 7.5% coating showed slight softening of coat but didn’t disintegrate in 0,1N Hcl. |
| |
| The results showed that 7.5% coating had a 76min disintegration time, 10% showed a 75min disintegration time, triple coat (TC) showed a 100 min disintegration time in pH 7.2 phosphate buffer. |
| |
|
Dissolution profile study
|
| |
| In vitro release studies were carried out over the formulated F01, F02 batches to ensure the ability of the coated formulations to protect the content against the stomach and intestinal environments (pH-7.2) and to release the drug in the colon, with various pH of 1.2 (0.1N Hcl), 6.0, 7.2 (phosphate buffer) the in vitro release study was carried out over the coated formulations of F01 and F02. |
| |
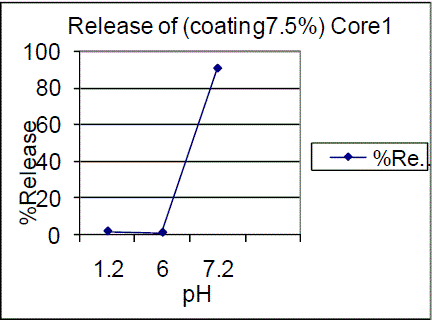
| The F01, F02 batch tablets coated with 7.5% of coating showed drug release of about 1.52%, 1.49% in 0.1N Hcl for 2 hrs. Which failed to comply with the USP standards of <1% in 0.1N Hcl. |
| |
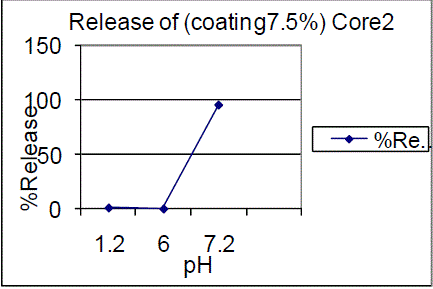
| On the other hand 10% coating of F01, F02 batches showed 0.73%, 0.61% in pH 1.2 and 0.51%, 0.99% in pH 6.0 and 82.65%, 91.73% of drug release in pH 7.2 |
| |
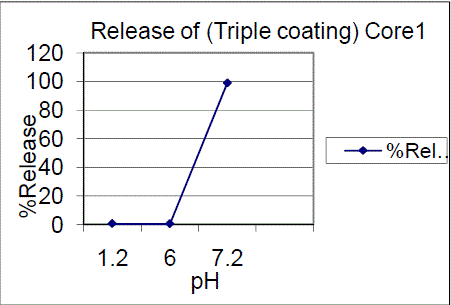
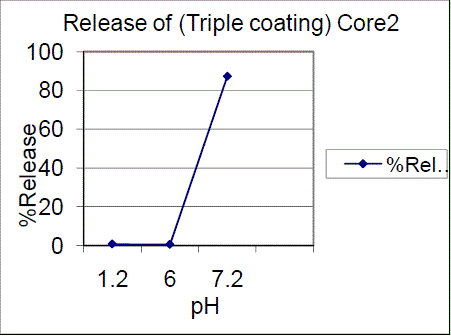
| Triple coating of F01, F02 batches had a release of 99.03%, 87.28% of drug release in pH7.2. The results showed that 7.5% failed to comply with the standards and 10%, triple coating of F01, F02 batches were passed, hence the 10%, TC is proved to be of great interest. Drug content for enteric coated tablets Standard Weight of Mesalamine taken (mg): 27.6 The drug content determined for the coated tablets were within the USP limits. |
| |
|
Stability data for mesalamine
|
| |
| Stability studies were carried out by exposing the core formulations F01, F02 to the temperatures of 8°c (CE), 25°c (RT), 50°c (VCE) for two months. Form the results of stability studies shows that there in no significant changes occurs in the drug content and weight of tablets. Thus it can be shown that the formulations were stable. |
| |
|
CONCLUSION
|
| |
| Colon targeted dosage forms have a distinct advantage over the existing conventional dosage forms. Mesalamine colon targeted dosage forms was prepared and coated by using Eudragit S100 and triple coat by Eudragit E100 and S100. Both formulations scarcely released mesalamine in pH 7.2 medium at 10% coating level, which indicate prepared formulations are suitable for the successful delivery of the drug into the colon and can be easily manufactured using conventional pharmaceutical coating technique and provided the promising candidate for specifically delivering drug to colon region, in particular for mesalamine 75mg in this study. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
 |
 |
 |
 |
 |
| Table 6 |
Table 7 |
Table 8 |
Table 9 |
Table 10 |
 |
 |
 |
| Table 11 |
Table 12 |
Table 13 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
|
| |