Irfan newaz khanA, marzina ajrinA, md. Razibul habib*B, maria islam khanA , md. Mominur rahmanB, mohammad shohelC
ADepartment of Pharmacy, University of Science & Technology Chittagong (USTC), Bangladesh
BDepartment of Pharmacy, East West University, Dhaka, Bangladesh
CInternational Islamic University Chittagong, Bangladesh,
DDepartment of Pharmacy, North South University, Dhaka, Bangladesh
- Corresponding Author:
- Mr. Md. Razibul Habib
Department of Pharmacy
East West University, Dhaka, Bangladesh.
Email: mrhjewel@gmail.com
Date of Submission: 02-05-2011 Date of Acceptance: 12-07-2011
Citation:Irfan Newaz Khan, Marzina Ajrin, Md. Razibul Habib, Maria Islam Khan, Md. Mominur Rahman, Mohammad Shohel,“Design, Physicochemical Evaluation And In Vitro Dissolution Studies Of Transdermal Patches Containing Aceclofenac”, Int. J. Drug Dev. & Res., Jul-Sep 2011, 3(3):233-241
Copyright: ©2010 IJDDR, Irfan Newaj Khan et al. This is an open access paper distributed under the copyright agreement with Serials Publication, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords
Aceclofenac, Transdermal Patch, PVP, Ethylcellulose
Introduction
Transdermal drug delivery is the delivery of drug across epidermis to achieve systemic effects. The success of transdermal patches lies in their commercialization. Transdermal patches control the delivery of drugs at controlled rates by employing an appropriate combination of hydrophilic and liophilic polymer [1-4]. The main components of transdermal patches are liner, drug, adhesive, membrane and backing.
There are four main types of transdermal patches are listed below:
1. Single-layer Drug-in-Adhesive
2. Multi-layer Drug-in-Adhesive
3. Drug Reservoir-in-Adhesive
4. Drug Matrix-in-Adhesive
At present, the most common form of delivery of drugs is the oral route. While this has the notable advantage of easy administration, it also has significant drawbacks - namely poor bioavailabiltity due to hepatic metabolism (first pass) and the tendency to produce rapid blood level spikes (both high and low), leading to a need for high and frequent dosing, which can be both cost prohibitive and inconvenient.The oral administration route is also complicated because of complications associated with gastrointestinal irritation, drug metabolism in the liver and is often impractical if a patient is vomiting or nauseous. For many medications it is important that the administration regime is as simple and non-invasive as possible in order to maintain a high level of compliance by a patient. Aceclofenac is rapidly and completely absorbed after oral administration, peak plasma concentrations are reached 1 to 3 hours after an oral dose. But the drug is highly protein bound (7.99%). The presence of food does alter the extent of absorption of Aceclofenac and the absorption rate is reduced. So the administration of physiologically active agents, such as Aceclofenac, through the skin ('transdermal drug delivery') has received increased attention because it not only provides a relatively simple dosage regime but it also provides a relatively slow and controlled route for release of a physiologically active agent into the systemic circulation. However, transdermal drug delivery is complicated by the fact that the skin behaves as a natural barrier and therefore transport of agents through the skin is a complex mechanism. The objective of the study was to Design & Development of Aceclofenac Transdermal Patch., Physiochemical studies of the developed patch, selecting chemical enhancer for improving the transdermal permeation of poorly absorbed drugs, Evaluating of the release kinetics of the drugs.
Experimental
Materials
Aceclofenac (Shanghai Shenxing Pharmaceutical Factory,China),polyvinyl alcohol (PVA;Hydrolysis- 98%;Ash-1%,BDH Chrmicals Ltd, Poole, England), Ethylcellulose(EC; Colorcon Asia Pvt. Limited,India), Di-n-butylphthalate (Assay ³ 98;Density:1.042- 1.045; Refractive index:1.492-1.494;Acidity: £0.1ml N% ;Merck Ltd, Mumbi),Chloroform(CHCl3=119.38g/mol; Purity:99- 99.4% VWR International Ltd, England),Nicotinamide(Potency:99.511%; DSM Nutritional Product, USA), Polyvinylpyrrolidone(PVP; BASF). All the chemicals were used as received without any further purification.
Development of Aceclofenac Transdermal Patch
Matrix – type transdermal patches containing Aceclofenac were prepared using the different ratios of PVP and EC by solvent evaporation technique in cylindrical both sides open glass molds. The bottom of the mold was wrapped with aluminum foil on
which the backing membrane was cast by pouring 4% w/v PVA solution followed by drying at 60°C for 6 h. The two polymers were weighted in requisite ratio and they were then dissolved in chloroform. di-nbutyl- pthalate 50% w/w of polymer composition was used as a plasticizer. The drug was added to the 40% w/w of the total weight of polymers, in the homogeneous dispersion, by slow stirring with a mechanical stirrer. The uniform dispersion (2ml each) was cast on the PVA backing membrane cast earlier and dried at 40°C for 6h.The backing membrane was then glued to a gummy tape keeping matrix side upward. The wax papers were used to give a protective covering. This was the final shape of the formulation. The dry patches were kept in desiccators until use [5].
Drug-Excipient interaction study
Drug-Excipient interaction study was performed using silica gel-coated TLC (Thin Layer Chromatography) plates and a mixture of one volume of hydrochloric acid, one volume of water six volume of glacial acetic acid and 11 volume of ethylacetate as mobile phase [6]. The TLC plates were prepared using slurry of silica-G. The prepared plates are activated at 110°C for 1.5 h. On the activated plates, 2 microliter of each solution in methanol containing (a) 12 mg/ml Aceclofenac containing different ratio of excipients, that is PVP, EC, Di-n-butyl phthalate were applied. The plates were dried in a stream of warm air for 10 minutes and then sprayed with ninhydrin solution. The plates were heated at 110°C for 15 min. The Rf values were calculated from the chromatogram obtained [7].
Physical characteristics of the prepared films Moisture Content
The prepared films were weighted and individually and kept in a desiccator containing activated silica at room temperature for 24 h. The films were weighted again individually until it showed a constant weight. The percentage of moisture content was calculated as a difference between initial and final weight with respect to final weight [7].
Moisture Uptake
A weighed film kept in desiccators at normal room temperature for 24 h was taken out and exposed to 84% relative humidity (saturated solution of potassium chloride) in desiccators until a constant weight for the film was obtained. The percentage of moisture uptake was calculated as the difference between final and initial weight with respect to initial weight [7].
Flatness
Longitudinal strips were cut out from each film, one from the center and two from either side. The length of each strip was measured and the variation in the length because of non-uniformity in flatness was measured by determining percent constriction, considering 0% constriction is equivalent to 100% flatness. [7]

Where 1 l = initial length of each strip and l 2= final length of each strip.
In-vitro Release – Dissolution Studies
The release – rate determinations is one of the most important studies to be conducted for all controlledrelease delivery systems. The dissolution studies of patches are very crucial, because one needs to maintain the drug concentration on the surface of stratum corneum consistently and substantially greater than the drug concentration in the body, to achieve a constant rate of drug permeation [8].
The dissolution of patches was performed using USP Basket Type Dissolution Apparatus. The patches An attempt was made at this point to learn whether the media phosphate buffer, pH 7.4, was able to maintain sink conditions in dissolution studies. E1% 1cm was 317.512 obtained from the solubility studies. Thus, phosphate buffer was chosen as the dissolution media because sufficient amount of drug dissolved in it (4–5 times the drug incorporated in patch), which is necessary to maintain sink condition.
| No. |
Formulation Code |
Ratio of PVP & EC
(mg) |
Total weight of
PVP & EC |
Chloroform (ml) |
Di-n-Butyl Phthalate |
Drug |
| 1 |
ACC1 |
1:3 |
500 |
5 |
50% w/w of polymer |
40% w/w of   polymer |
| 2 |
ACC 2 |
2:2 |
500 |
5 |
50% w/w of polymer |
40% w/w of   polymer |
| 3 |
ACC 3 |
3:5 |
500 |
5 |
50% w/w of polymer |
40% w/w of   polymer |
Table 1: Composition of Prepared Patch
| Formulation code |
PVP/EC |
Rf Value |
| Drug |
Drug-
Excipient |
| ACC1 |
1:3 |
0.709 |
0.756 |
| ACC2 |
2:2 |
0.793 |
0.793 |
| ACC3 |
3:5 |
0.794 |
0.828 |
Table 2: Determination of Drug-Excipient Interaction using the TLC method
The physicochemical studies like moisture content, moisture uptake, flatness etc provide information regarding the stability of the formulations. The moisture content and moisture uptake (Figure 1, 2) varied to a small extent in all the formulations were placed in respective baskets with their drug matrix exposed to phosphate buffer, pH 7.4. All dissolution studies were performed at 320C, at 50 rpm, with each dissolution jar carrying 900 ml of buffer. Samples were withdrawn at different time intervals and analyzed using a UV spectrophotometer at 273 nm against blank, Cumulative amounts of drug released was plotted against time for different formulations [8].

Figure 1: Percentage of moisture content from Aceclofenac containing different matrix films

Figure 2: Percentage of moisture uptake from Aceclofenac containing different matrix films prepared by using different ratios of PVP & EC with nicotinamide. Data shows mean ± SE (n = 6).
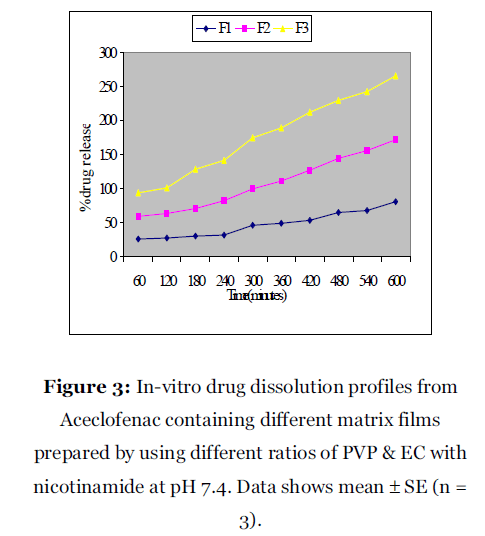
Figure 3: In-vitro drug dissolution profiles from Aceclofenac containing different matrix films prepared by using different ratios of PVP & EC with nicotinamide at pH 7.4. Data shows mean ± SE (n = 3).

Figure 4: Higuchi plot for release profiles of Aceclofenac containing different matrix films prepared by using different ratios of PVP & EC with nicotinamide at pH 7.4. Data shows mean ± SE (n = 3).

Figure 5: First order plot for release profiles of Aceclofenac containing different matrix films prepared by using different ratios of PVP & EC with nicotinamide at pH 7.4. Data shows mean ± SE (n = 3).
Results
TLC studies were performed to assess any interaction between the drug and excipients. The data obtained suggested that there was no interaction between the drug and the excipients because the Rf values of both the drug and the drug– excipient solutions were nearly similar (Table 2). However, there was increased moisture content with an increase in hydrophilic polymers. The results of moisture uptake studies for different formulations were very unusual as it shows some negative values may be due to the presence of nicotinamide, which prevent the moisture uptake, and the effect was loss of weight. The results of the flatness study showed that some of the formulations had very little differences in the strip lengths before and after their cuts. It indicates almost very near to 100% flatness observed in the formulated patches. Thus, a very little amount of constriction was observed in the film of different formulations and it indicates smooth flat surface of the patches. A 100% flatness of all the formulations indicates (Table 4) no amount of constriction in formulated transdermal membrane strips. Dissolution studies are important for ensuring the sustained release performance and the reproducibility of rate and duration of drug release. It was observed that as the concentration of hydrophilic polymer, PVP, increased in the formulations, the rate of dissolution increased subsequently and the best result found in the polymer ratio 3:5.
| Formulation code with nicotinamide |
Ratio of PVP/EC |
% Drug content |
| ACC1 |
1:3 |
98.3 ± 0.4 |
| ACC2 |
2:2 |
99.1 ± 0.3 |
| ACC3 |
3:5 |
99.6 ± 0.1 |
Table 3: % Drug content in the prepared patches
| Formu lation Code |
Polymer Ratio(P VP: EC) |
% Flatness |
Thickness (Cm) |
| ACI-1 |
1:3 |
100.00 |
0.00800 |
| AC1-2 |
99.99 |
0.00800 |
| AC2-1 |
2:2 |
99.98 |
0.00850 |
| AC2-2 |
99.87 |
0.00900 |
| AC3-1 |
3:5 |
99.88 |
0.00850 |
| AC3-2 |
100.00 |
0.00850 |
| Mean |
99.95333 |
0.00833 |
| SD |
0.052203 |
0.000549 |
Table 4: Data for % Flatness & Thickness (Cm) uniformity of the films
To study the drug content into the patches a 5-cm2 film was cut into small pieces, put into a 100-mL buffer (pH 7.4), and shaken continuously for 24 hours. Then the whole solution was ultrasonicated for 15 minutes. After filtration, the drug was estimated spectrofluorometrically at an excitation wavelength of 240 nm and an emission wavelength of 340 nm. The preliminary studies indicated that there was no interference of polymers in the excitation and emission wavelengths of the drug.
Folding endurance was determined by repeatedly folding the film at the same place until it broke. The number of times the film could be folded at the same place without breaking was the folding endurance value. Folding endurance test results indicated that the patches would not break and would maintain their integrity with general skin folding when applied. The results of skin irritation tests of the Aceclofenac transdermal patches in comparison with formalin (0.8%) showed that the transdermal systems induced negligible erythema and edema, but formalin induced severe erythema and edema. Formalin induced high grade of irritation, indicated by ‘severe’ inflammation and edema besides showing discontinuity in epidermis, thin epidermis, ulceration and hyperplasia. The skin irritation test of the transdermal formulations ACC3 (PVP: EC: 3:5) with nicotinamide showed a skin irritation score (erythema and edema) of less than 2 (Table 5). According to Draize et al, compounds producing scores of 2 or less are considered negative (no skin irritation). Hence, the developed transdermal formulations are free of skin irritation.
| Rat No. |
Control |
ACC3 (with Nicotinamide) |
Formalin |
| |
Erythema |
Edema |
Erythema |
Edema |
Erythema |
Edema |
| 1 |
0 |
0 |
0 |
1 |
2 |
2 |
| 2 |
0 |
0 |
1 |
0 |
3 |
1 |
| 3 |
0 |
0 |
1 |
0 |
3 |
2 |
| 4 |
0 |
0 |
2 |
1 |
2 |
3 |
| 5 |
0 |
0 |
1 |
0 |
3 |
3 |
| 6 |
0 |
0 |
2 |
0 |
3 |
2 |
| Average |
0 |
0 |
1.17 ±0.3073 |
0.33 ±0.2108 |
2.67 ±0.2108 |
2.16 ±0.3073 |
Erythema scale: 0, none; 1, slight; 2, well defined; 3, moderate; and 4, scar formation. Edema
scale: 0, none; 1, slight; 2, well defined; 3, moderate; and 4, severe. Table 5: Skin Irritation Scores Following Transdermal Patch Administration.
Table 5: Skin Irritation Scores Following Transdermal Patch Administration.
Discussion
In this study, it was desired to design a TDDS of Aceclofenac using a polymeric matrix film. This allows one to control the overall release of the drug via an appropriate choice of polymers [9] and their blends studied here, utilizing the different diffusion pathways created due to the blend of polymers to produce overall desired steady and sustained drug release. Cumulative amounts of drug (Aceclofenac) released per cm2 from the different TDDS of varied ratio of PVP and EC showed variable release patterns (Fig 3, 4, 5). The process of drug release in most of the controlled/sustained release devices including transdermal patches is governed by diffusion [10]. When this matrix patch comes into contact with an in vitro study fluid, thermodynamically compatible with the polymer, the fluid is absorbed into the polymer matrix and this initiates polymer chain dissolution process in the matrix [11-12]. When the active agent (drug) is released from the matrix in such a way that the rate of release of the drug remains constant, the release kinetics of the drug are believed to follow a zeroorder kinetics [13]. The release kinetics was studied to identify the best possible release mechanism of the drug. The percentage of Aceclofenac was plotted against time to get the zero order plots (Fig 3). In addition the percentage of release was plotted against Square Root of Time in Minutes to get higuchi plot (Fig 4). Again the log remaining was plotted against time to get the first order plot (Fig 5). The higuchi plot showed reasonably straight line with high correlation coefficient.
A 100% flatness of all the formulations indicates (Table 4) no amount of constriction in formulated transdermal membrane strips. Thus this does not constrict when it is applied on to the skin. On the other hand, thicknesses were also measured to understand the content uniformity & found satisfactory. The moisture content in the formulations was related with the ratio of PVP & EC. Moisture content was increased with the increment of hydrophilic polymer, PVP and it was highest at the ratio of 3:5. Moisture contents in the other formulations were found to be low. The results of moisture uptake studies for different formulations were very unusual as it shows some negative values may be due to the presence of nicotinamide, which prevent the moisture uptake, and the effect was loss of weight.
Aceclofenac containing different matrix films prepared by using different ratios of PVP & EC with nicotinamide at pH 7.4. Data shows mean ± SE (n =
Conclusion
The moisture content in the formulations was related with ratio of PVP and EC.It was observed that as the concentration of hydrophilic polymer, PVP, increased in the formulations, the rate of dissolution increased subsequently and the best result found for polymer ratio 3: 5. The release kinetics was also studies to identify the best possible release mechanism of the drug by different types of plot like Zero order plot, Higuchi plot, First order plot. From study of skin reaction it was also found that there was no significant reaction was developed during the contact of patch with dermis, only a slight erythema and edema were developed when contacted with skin for 24 hours. So no or little irritation was observed. Finally from the study it was found that Aceclofenac could be given as Transdermal Patch and further In vivo and In vitro investigations are required.
Conflict of Interest: NIL
Source of Support: NONE
5650
References
- Keith AD. Polymeric matrix considerationnfor transdermal devices. Drug Dev IndnPharm 1983; 9: 605-621.
- Chein YW. Development of transdermalndrug delivery system. Drug Dev Ind Pharm 1983; 9: 589-651.
- Misra AN. Transdermal drug delivery in:nJain NK. Contrilled and Novel DrugnDelivery, Varghese Publication, New Delhi,n1988, pp100-129.
- Walters KA. Transdermal drug delivery:nsystem design and composition in:nSwarbrick K, Boylan JC. Encyclopedia ofnPharmaceutica Technology, Marcel Dekker,nNew York, NY, 1999, pp 306-320.
- Arora P, Mukherjee B. Design, development,nphysicochemical and in vitro and in vivonevaluation of transdermal patchesncontaining diclofenac diethylammoniumnsalt. J Pharm sci 2002; 91: 2076-89.
- British Pharmacopoeia, Vol II. London, UK:nThe British Pharmacopoeia Commission,nThe Stationery Office; 1999, pp 493–494.
- Baichwal RW. Advances in drug deliverynsystems. Bombay: MSR Foundation, 1983,npp 136-47.
- Fang JY, Wang RJ, Huang YB, Wu PC, TasinYH. Passive and ionotophoretic delivery ofnthree diclofenac salt across various skinntypes. Biol Pharm Bull 2000; 23:1357-62.
- Sarpottdar PP, Gaskill J, Giannini RP. Effectnof polyethylene glycol 400 on thenpenetration of drugs through humanncadaver skin in-vitro. J Pharm Sci 1986; 75:n26-28.
- Siepmann J, Ainaoui A, Vergnaud JM,nBodmeier R. Calculation of dimensions ofndrug polymer devices based on diffusionnparameter, J Pharm Sci 1998; 87: 827-32.
- Brochard F, Gennes PG. Kinetics of polimerndissolution, physicochem. Hydrodynamn1983 ;4: 313-322.
- Bonferoni MC, Caramella C, Sangalli ME,nConte J, Hernandez RM, Pedraz JL.nRheological behavior of hydrophilicnpolymers and drug release from erodablenmatrices. J Cont Rel 1992; 18: 205-12.
- Martin A, Bustamante P, Chun AHC.nPhysical pharmacy, 4th ed., B.I. Wavery pvt.nLtd. New Delhi, 1999. pp 284-317.











