Keywords
Destained slides; Periodic acid schiff stain; Candidiasis; Papanicolaou stain
Introduction
Vulvovaginal Candidiasis (VVC) is an infection caused by candida species that affect millions of women every year [1]. It is considered as the second most common cause of Vaginitis [2]. It is estimated that approximately three out of four healthy adult women experience at least one episode of VVC during their lives, most commonly when they are of child bearing age [3]. Information of its incidence is incomplete and hampered by inaccuracies of diagnosis. When VVC is left untreated, many complications have been appointed as its consequence. Schwiertz et al., reported 77% rate of misdiagnosis of VVC by physicians is based on clinical evidence alone [4]. According to Odds, the diagnosis of candidiasis cannot be made adequately only by clinical findings. It must be based on the presence of clinical signs allied to observation of hyphae or Pseudo hyphae at cytopathology [5]. Smears with candidiasis Often show Cellular changes that resemble epithelial atypia and patient may be asymptomatic. This necessitates accurate identification of candida, especially for those who are at risk of reinfection [6].
PAP smear is the most common method of routine cytological examination. But it is an insensitive method for candida diagnosis; yielding positive results in only about 25% of patients with culture positive symptomatic VVC [2]. Due to the small size of candida and low colour range of PAP smear images identification of candida requires precision, patience and lots of experience especially when organisms were less in number. The presence of blood, inflammatory cells, mucus and other debris makes the detection process unreliable. When the organisms are good in number in well-stained PAP smear, identification of candida species is not that difficult and a need for PAS also does not arise [7].
Demonstration of Candida species by Periodic acid Schiff stain unquestionably remains the mainstay of identification of fungus in routine cytological preparations [8]. It stains myriad of carbohydrate containing substances like glycogen, fibrin, amyloid, certain sialo and sulphomucins and fungal cell walls [9]. The comparative analysis demonstrated higher prevalence of candida species in PAS than in Gram staining and PAP staining [10,11]. The PAS staining method is more sensitive than haematoxylin and eosin sections (poor color contrast with their surroundings) or cultural methods in tissue sections [12]. Increased visibility in PAS is related to the specific site in the candida cell wall, which takes up brilliant magenta color due to rich glycogen content. This makes the observer to identify candida easily in the background of lightly stained epithelial cells.
Although PAS is considered the best method for identifying candida species, it is little used for its higher cost in comparison with other histochemical staining methods [13]. Other major drawback for its use in cytology practice is highly variable sample quality among slides. Smears often differ in cellularity, contaminants, individual cell population and morphology. Typically use of Periodic Acid Schiff stain is done after the review of routine Papanicolaou stained smear and thus requires an additional unstained smear. If the additional sample lacks the representative material, false negative or inconclusive results may occur [14].
The present study was designed to determine the use of destained slide for application of Periodic Acid Schiff stain, for candida species and to determine whether destaining slides after papanicolaou staining is a reliable option for diagnosis of candidiasis in cytology smears.
Materials and Methods
Cervical smears positive for Candida organisms were retrieved from archives of cytology department of our university medical College and hospitals, after obtaining approval from the Institutional Standard Research Review Board (Approval no: SRB/ SDMDS16 OMP/02). 12 cases were selected. The smears had been stained with Papanicolaou stain and mounted with DPX mounting medium. All slides were photographed with Magnus digital camera (fitted on Olympus microscope) in recorded positions on x and y-axes. Minimum of 4 fields per slide using 10x, 40x were taken.
The slides were placed in xylene for one to two days to remove the Coverslips (Figure 1). After coverslip removal (Figure 2) they were left in xylene for one additional day to ensure complete removal of mounting medium. Then slides were divided into three groups randomly (4 slides in a group) and they were destained carefully using three different concentrations of Acid alcohol; 1%, 5%, 10% (i.e. 1, 5 and 10 ml of concentrated Hydrochloric acid in 70% ethanol) respectively (Figure 3). Slides were ensured destained (colorless) by viewing under the microscope (Figure 4). Following which, all the slides were hydrated in decreasing grades of alcohols (100%, 70%, and 50%), tap water and distilled water (2 minutes each) (Figure 5).

Figure 1: Destaining process.
Figure 2: Coverslip removal with xylene.

Figure 3: Destaining with 1%, 5% and 10% acid alcohol.

Figure 4: Destained slide and its microscopic appearance showing complete destained process.

Figure 5: Hydration with decreasing grades of alcohol (100%, 70% and 50%).
Then they were restained with periodic acid stain and haematoxylin counter stains. Slides were photographed on same positions as for Papanicolaou stained smear and were evaluated qualitatively by an observer blinded to the diagnosis using an observer score sheet. The observer assessed candida demonstration, staining intensity, cell morphology, nuclear counterstaining and presence of nonspecific (background) staining of the slide. Each parameter was scored from 0 to 3 based on their magnitude, which ranged from poor, acceptable, good to excellent respectively. In case of in the case of Background Staining Score 0 meant insignificant background stain, score 1 that of Acceptable grade, score 2 of Unacceptable grade, and score 3 signified nonspecific stains that affected Diagnosis. The collected data were tabulated and analyzed using SPSS statistics version 21.0.0.0. The diagnosis of candidiasis was made only when characteristic fungal cell morphology was identified.
Result
Destaining was achieved using all three concentrations (1%, 5%, and 10%) of acid alcohol. No differences in the cell morphology were noted. The time taken by 5% acid alcohol to completely destain the slides was 10-15 minutes on an average (depending on thickness of the smear). While 10% acid alcohol took lesser time (5 minutes), 1% acid alcohol took minimum a day to destain the slides completely.
Periodic acid Schiff stained fungal elements positively in all the twelve destained smears. The Candida species were demonstrated readily as tangled magenta masses of elongated hyphae and pseudohyphae with numerous budding yeast cells. Thus they were diagnosed easily in the background of lightly stained epithelial cells compared to original Papanicolaou smear (Figure 6). Staining intensity was 50% excellent and 50% good. Candida demonstration was excellent in 75% cases, Acceptable in 17% cases, Good in 8% cases. Nuclear counterstaining was 50% acceptable and good 42%, excelled in one case (8%). cell morphology was only acceptable in 33%, good in 17%, excellent in 50% of cases. Nonspecific stains were present in acceptable level in 42% cases, whereas it was unacceptable in 25% cases, affected the diagnosis in one case (8%). (Table 1 and Graphs 1-5).
| Parameters |
Poor
(Cases in %) |
Acceptable
(Cases in %) |
Good
(Cases in %) |
Very good
(Cases in %) |
Candidial
demonstration |
-- |
17% |
8% |
75% |
Nuclear
counterstain |
-- |
50% |
42% |
8% |
| Cell morphology |
-- |
33% |
17% |
50% |
| Stain intensity |
-- |
-- |
50% |
50% |
| -- |
Affected diagnosis |
Unacceptable |
Acceptable |
Non
significant |
Background
stain |
8% |
25% |
42% |
25% |
Table 1: Representing results with various parameters in the column and row.
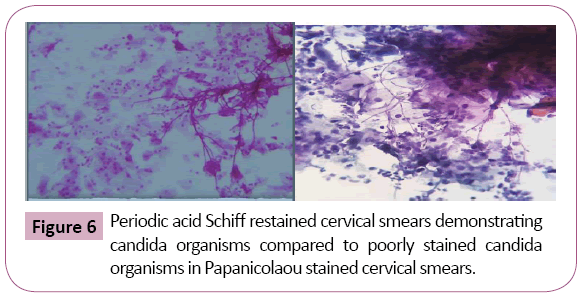
Figure 6: Periodic acid Schiff restained cervical smears demonstrating candida organisms compared to poorly stained candida organisms in Papanicolaou stained cervical smears.
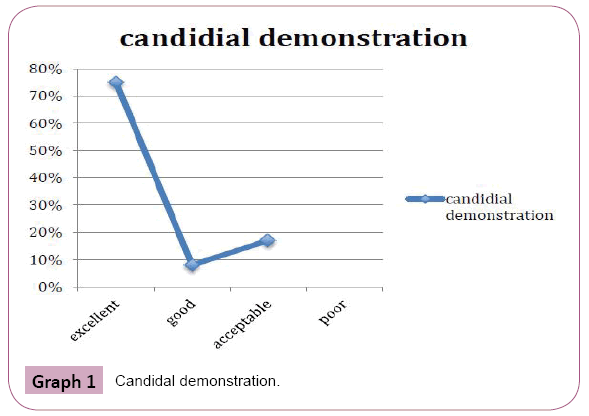
Graph 1: Candidal demonstration.
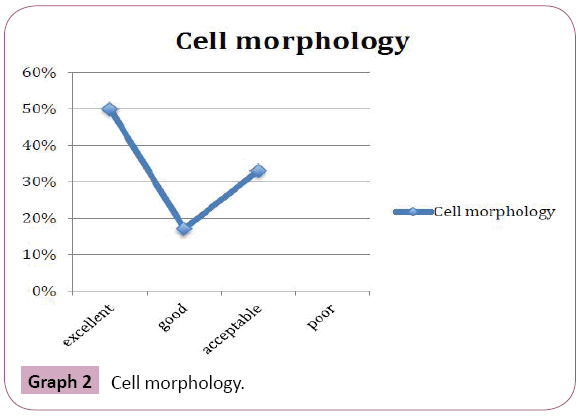
Graph 2: Cell morphology.
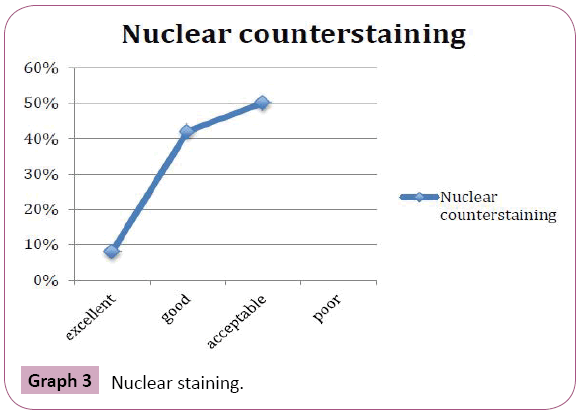
Graph 3: Nuclear staining.
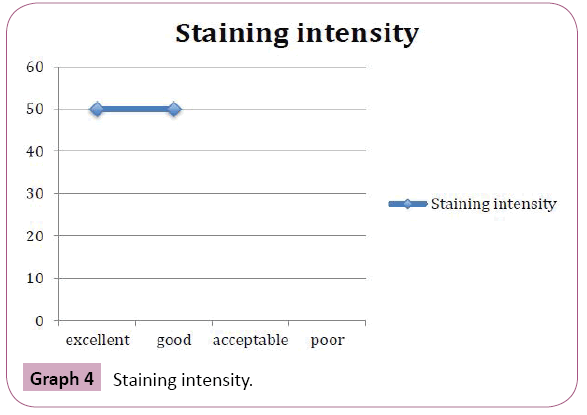
Graph 4: Staining intensity.
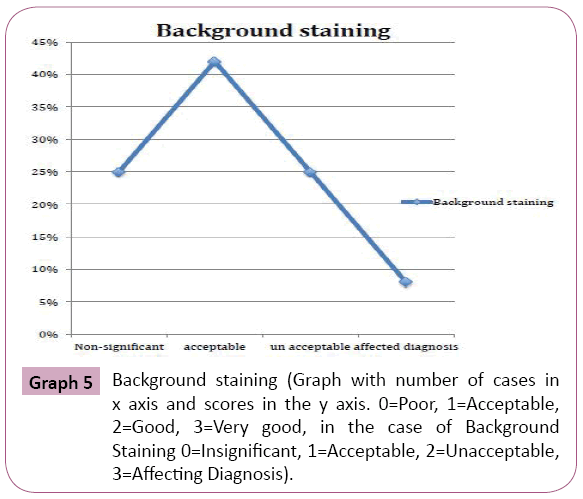
Graph 5: Background staining (Graph with number of cases in x axis and scores in the y axis. 0=Poor, 1=Acceptable, 2=Good, 3=Very good, in the case of Background Staining 0=Insignificant, 1=Acceptable, 2=Unacceptable, 3=Affecting Diagnosis).
Discussion
Destaining is not a commonly used procedure. Only isolated reports recommend destaining slides when special stains are required namely when no unstained smears remain or any suspected feature has been observed in a single slide [14]. In the present study, we have used the cases with single slide showing areas, suspicious for candida. Thus destaining was done to use the slides to confirm diagnosis by application of the special stain. Although all three concentrations (1%, 5% and 10%) of acid alcohol achieved the destaining process, 5% and 10% acid alcohol were faster. While the slides destained with 5% acid alcohol were uniformly colorless and better (especially in case of thicker smears), destaining with 10% acid alcohol was in patches and not uniform throughout the slide. Thus we recommend using 5% acid alcohol to obtain uniform, faster destaining of smears. This is in accordance with results of Marcos et al. [14] who also compared 1%, 5% and 10% acid alcohol for destaining hemacolour-stained smears of cats and dogs and found that 5% acid alcohol produced optimal destained smears.
A Particular problem encountered during destaining process was detachment of smears from slides soon after they were placed in acid alcohol for destaining from xylene. ‘Fixing’ the smears in alcohol before destaining procedure helped to prevent this problem.
On examination of Periodic Acid Schiff restained smears, an expert has evaluated various parameters, which are commonly reported to be affected by destaining process. Candida was demonstrated successfully irrespective of destaining process as Eosinophilic yeast form/true hyphae form/long pseudo hyphae form along with tangles & skewers of squamous cells around them in all the twelve smears compared to the poorly highlighted organisms in Papanicolaou stain. Moreover, when organisms were less in number, it was difficult to demonstrate them with papanicolaou stain. In a study by Kumaraswamy Naik et al. [8] where Periodic acid schiff stain, Papanicolaou stain and culture were compared for detection of oral candida species, PAS stained smears were concluded as more reliable with sensitivity=100% and specificity=66.7% compared to light and fluorescent microscopy of PAP stained smears (sensitivity=89.3%, specificity=16.7%) and culture (90.30% positive). In a comparative analysis by Padilha [11], higher prevalence of candida spp. was demonstrated in PAS than in Gram’s staining and PAP staining.
Compared to Papanicolaou stained smear, morphology of the squamous epithelial cells were only acceptable in 33.3% of cases and only 8% showed good nuclear staining. Even the well-stained smears were slightly blurry, impairing analysis of cell morphology. This can be attributed to the nature of the stain as PAS is not a cytoplasmic or nuclear stain and works on contrast method. Similar results were shown by Almeida et al. [15] where Morphology of squamous cells and their nucleus were in only acceptable level in 33.3% of cases. PAS resulted in unclear, shadowed staining of cytoplasmic structures and the nuclei. Although fungi are well demonstrated by PAS staining, the method does not permit adequate analysis of epithelial cells for dysplastic changes. Papanicolaou is the method most widely used in exfoliative cytology because it permits demonstration of difference between cells of various epithelial layers, enables evaluation of cytoplasmic and nuclear characteristics of epithelial and inflammatory cells in precision of details. This is in agreement with its indication for early detection of carcinoma. As candidiasis can frequently co-exist with dysplastic changes, combination of Papanicolaou and PAS staining method is ideal for the cytological diagnosis of candidiasis.
In our study smears stained with Periodic Acid Schiff did not stain bacteria (Bacterial organisms took up haematoxylin along with inflammatory cells and other background substances). This was similar to the observations of Almeida et al. [15] who inferred that with respect to evaluation of morphological identification of bacteria and fungus, PAS gave excellent staining of fungi but weak staining of bacteria whereas PAP produced good staining of bacteria and weak staining of fungi, whereas thus PAS identified. Starr et al. [12] categorized PAS method as one with reliable exclusion-potential as it demonstrated only the fungal agents. Thus this identified fungus in precision compared to Papanicolaou stain. In some cases, poorly expressive fungal flora might go unnoticed if stained by papanicolaou resulting in false negative diagnosis. In this case, PAS permits permits identification of fungus and diagnostic confirmation.
Destaining has been reported to have effect on stain uptake (staining intensity) by smears on restaining. In our study, 50% of cases showed appropriate stain Intensity and in 50% of cases, the cells in the smear were darkly stained uniformly although candida was well delineated with its distinct morphology. Similar results were observed in Marcos et al., study [14] and he recommends use of sodium hypochlorite to remove the excess of stain taken up by cells so that the organism gets highlighted.
Non-specific, background staining was observed in 75% of the cases (9 out of 12 smears). Mucus, neutrophils, macrophages and artifacts also stained magenta with PAS stain and interfered with ease with which candida was diagnosed. In one case, all the cells uniformly took up PAS hindering the diagnosis although candida could be well delineated by morphology. Starr et al. [12] describes Artefacts as constant problem encountered during search for fungi in his study. It was largely reflective of the lack of specificity of the PAS stain as it mainly works on contrast method and is not a specific histochemical procedure. Marcos et al. [14] suggests Predigestion with diastase enzyme before periodic acid Schiff staining (reduce nonspecific staining of nonfungal material or cells) or additional restaining procedures with GMS (by destaining PAS stain) for avoiding misinterpretation of PASpositive materials as fungi.
Although PAS has been used previously in destained cytological samples from humans in Kumar et al. [16], where it was recommended for the diagnosis of cryptococcosis, when few or thin-walled organisms are present, we confirmed in our study that destaining could be a useful procedure in diagnosing candidiasis when limited material or previously stained material are available.
Conclusion
PAS staining is not affected by destaining procedures in cervical smears. Destaining can be a valuable choice when only stained slides are present or any interesting feature is observed in a particular slide.
23370
References
- Goncalves B, Ferreira C, Tiago Alves C, Henriques M, Azeredo J, et al. (2016) Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit Rev Microbiol 42: 905-927.
- Geiger AM, Foxman B, Gillespie BW (1995) The epidemiology of vulvovaginal candidiasis among university students. Am J Public Health 85: 1146-1148.
- Schwiertz A, Taras D, Rusch K, Rusch V (2006) Throwing the dice for the diagnosis of vaginal complaints? Annals of Clinical Microbiology and Antimicrobials 5(1): 4.
- Odds FC (1994) Pathogenesis of candida infections. J Am Acad Dermatol 31: 2-5.
- Nyirjesy P, Alexander AB, Weitz MV (2005) Vaginal Candida parapsilosis: pathogen or bystander? Infect Dis Obstetrics Gynecol 13: 37-41.
- Momenzadeh M, Sehhati M, Mehri Dehnavi A, Talebi A, Rabbani H (2017) Automatic diagnosis of vulvovaginal candidiasis from pap smear images. J Microsc 267: 299-308.
- Kumaraswamy Naik LR, Shetty P, Krishna Prasad MS, Karnaker VK, Shroff SE, et al. (2016) Fluorescence of candida in diagnosis of oral candidiasis. Indian J Dent Res 27: 618-622.
- Bancroft JD (1967) An introduction to histochemical technique. London: Butterworth & Co pp: 62-175.
- Og A, Oe O, To A (2010) Sensitivity of a papanicolaou smear in the diagnosis of candida albicans infection of the cervix. North American journal of medical sciences 2(2): 97.
- Padilha CML, Picciani BLS, Santos BMd, Silver Junior A, Dias EP (2014) Comparative analysis of grams method and PAS for the identification of candida spp. Samples from the oral mucosa. J Bras Patol Med Lab 50: 352-358.
- Starr GF, Dawe CJ, Weed LA (1954) Use of periodic acid Schiff stain in identification of pathogenic fungi in tissues. Am J Clin Pathol 25: 76-83.
- Sandrin R, Og A, Oe O (2010) A Sensitivity of papanicolaou smear in the diagnosis of candida albicans infection of the cervix. N Am J Med Sci 2: 97-99.
- Macros R, Santos M, Santos N, Malhao F, Ferreira F, et al. (2009) Use of destained cytology slides for the application of routine special stains. Vet clin Pathol 38: 94-102.
- Almeida JD, Lima CF, Brandao AA, Cabral LA (2008) Evaluation of staining methods for cytologic diagnosis of oral lesions. Acta cytol 52: 697-701.
- Kumar P, Saran RK, Gondal R, Malhotra V (2005) Smear morphology of cryptococcosis presenting as a subcutaneous swelling in healthy adults: A report of three cases. Cytopathology 16: 143-146.

















