Key words
|
| MSDE; Primary malignant brain tumor; MRI; Dissemination |
Introduction
|
| Several types of primary malignant brain tumors show infiltrative invasion characteristics into normal brain parenchyma and dissemination into the subarachnoid space. These tumors also have invisible microinvasion. Therefore, tumor recurrence after resection is common and it is associated with a poor prognosis [1,2]. It is important to accurately diagnose the range of infiltration and dissemination in these tumors using MRI sequences with a contrast medium. These lesions are so small that 3 dimensional T1 sequences with contrast medium such as T1 turbo field echo (T1TFE) is useful. However, it is known that the enhancement of 3-dimensional (3D) T1TFE with contrast medium (3D-CE-T1TFE) is lower than for that of conventional spin echo with contrast medium (SE-CE-T1WI), which is not a 3D MRI sequence [3]. |
| Multisection motion sensitized driven equilibrium (MSDE) is a new MRI technique similar to diffusion weighted imaging (DWI); MSDE is used to evaluate atherosclerotic plaques in the coronary and carotid arteries. This method uses a preparative sequence, a diffusion pulse with a very low b-value consisting of three nonselective RF pulses with flip angles of 90-180-90 degrees and symmetric gradients around the 180° pulse. MDSE causes phase dispersion of blood spin using a magnetic field gradient and suppresses the blood flow signal, and the 3D T1 or T2 weighted images can thus be acquired [4,5]. The contrast media can be used and abnormal enhanced lesions can be evaluated if MSDE with T1 weighted images is used. Therefore, 3D MSDE with contrast medium (3D-CE-MSDE) has also been used to detect brain metastasis because it clearly shows only abnormal enhancement in brain parenchyma with no vascular signal [6]. 3D-CE-MSDE was shown to be useful in detecting a case of malignant glioma [7]. We hypothesized that 3D-CE-MSDE also may be able to visualize only the abnormal enhancement of infiltration to brain parenchyma and dissemination into the subarachnoid space because it can suppress any vascular signal including medullary veins. The purpose of this study is to systematically evaluate the abnormal enhancement and to determine the usefulness of 3D-CE-MSDE for primary malignant brain tumors. |
Materials and Methods
|
| This protocol was approved by the ethics committee at our institution. We declare that all human and animal studies have been approved by our ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study. |
| Subjects |
| The subjects were prospectively 29 consecutive patients diagnosed with primary malignant brain tumors at our university hospital from November 2012 to January 2014 (mean age: 56.2 ± 25.2 years; 13 males and 16 females). Neurosurgeons and/or pathologists diagnosed these patients clinically and/ or pathologically. The diagnoses included 15 glioblastoma multiformes (GBM) including 1 strongly clinically suspicious case and 1 recurrent case, 8 anaplastic astrocutomas including 1 recurrent case, 2 anaplastic oligodendrogliomas, 2 central nervous system malignant lymphomas, 1 germinoma and 1 medulloblastoma. |
| Image acquisition |
| Both 3D-CE-T1TFE and 3D-CE-MSDE were performed as a preoperative study on the same day in all the patients. If 3D-CET1TFE was initially performed in the first patient, 3D-CE-MSDE was initially performed in the next patient in order to take into consideration the time lag from administration of the contrast medium. We used a gadolinium-based contrast medium (gadopentetate dimeglumine, 0.1 mmol/L, Bayer HealthCare, Japan). |
| We used a 3.0 Tesla MRI unit (Intera Achieva 3.0T, Philips, The Netherlands). The parameters for each sequence are as follows: 1) 3D-CE-T1TFE: TR/TE, 11/6.3 ms; ACQ voxel size: 0.79 × 0.79 × 0.80 mm; REC voxel size: 0.47 × 0.47 × 0.80 mm; NEX: 1; field of view (FOV): 240 mm; flip angle: 8 degrees; slices: 200; slice thickness: 0.80 mm; coil: SENSE-Head-32ch; and scan time: 6 min, 33 sec; and 2) 3D-CE-MSDE: TR/TE: 5.2/2.5 ms; ACQ voxel size: 0.79 × 0.77 × 1.25 mm; REC voxel size: 0.75 × 0.75 × 1.25 mm; NEX: 1; FOV: 240 mm; flip angle: 20 degrees; b-value (flow velocity encoding 1 cm/s): 1.45; slices: 60; slice thickness: 1.25 mm; coil, SENSE-Head-8ch; and scan time: 4 min, 39 sec. |
| Image analysis |
| The contrast-to-noise ratio (CNR) was initially calculated as indicated below for both 3D-CE-T1TFE and 3D-CE-MSDE. The “lesion” was considered the main tumor. If the main tumor was unclear because of hemorrhage or for any other reason, we measured the largest satellite-enhanced lesion near the main tumor. “Normal-parenchyma” is defined as normal signal intensity parenchyma on T2 weighted images. “Disseminations” is defined as spotty enhancement in the subarachnoid space. We statistically analyzed each result using a paired t-test (IBM SPSS Version 22.0 statistics). |
| CNR = (SIlesion– SInormal-parenchyma)/0.5(SDlesion– SDnormal-parenchyma) |
| SI: Signal Intensity, SD: Standard Deviation |
| Each acquired image was separately evaluated by two radiologists (readers A and B, with 5 and 9 years of experience, respectively). They observed the images from three directions (axial, coronal and sagittal images) using a 3D workstation (Aquarius Net, TeraRecon, Inc., San Mateo, CA, USA). They separately evaluated the number of intraaxial enhanced lesions and disseminations that were visible in each sequence. Two radiologists had never seen the subjects. If they evaluate 3D-CE-T1TFE in one day, they don’t evaluate 3D-CE-MSDE in the same day in the same patient and they evaluate another sequence after approximately one week. Images were read within 10 minutes of acquisition for each sequence to ensure equal reading conditions. We analyzed each result using a paired t-test and p<0.05 was considered significant for all analyses. |
Results
|
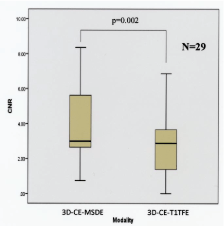
| The results are summarized in Figures 1-3. The CNR for 3D-CEMSDE was significantly higher than that of 3D-CE-T1TFE (p=0.002; Figure 1). |
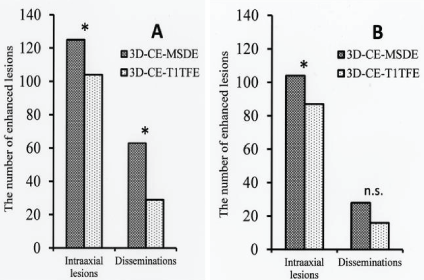
| Radiologist A detected 104 intraaxial lesions for 3D-CE-T1TFE and 125 intraaxial lesions for 3D-CE-MSDE, and also 29 disseminations for 3D-CE-T1TFE and 63 disseminations for 3D-CE-MSDE. Radiologist B detected 87 intraaxial lesions for 3D-CE-T1TFE and 104 intraaxial lesions for 3D-CE-MSDE, and also 16 disseminations for 3D-CE-T1TFE and 28 disseminations for 3D-CE-MSDE. |
| For radiologist A, 3D-CE-MSDE was superior to 3D-CE-T1TFE for detecting both intraaxial lesions and disseminations (p=0.04 and p<0.01, respectively; Figure 2A). For radiologist B, 3D-CEMSDE was superior to 3D-CE-T1TFE in detecting intraaxial lesions (p=0.01). However, for disseminated lesions, no significant difference in detectability was observed (p=0.097; Figure 2B). |
Discussion
|
| We revealed that the 3D-CE-MSDE is superior to the conventional 3D-CE-T1TFE in detecting intraaxial enhanced lesions and disseminations for primary malignant brain tumors in this study and 3D-CE-MSDE is the useful method in diagnosing primary malignant brain tumors. MSDE causes phase dispersion using a preparation pulse similar to DWI. Therefore, MSDE can completely suppress any flow signal. MSDE has been applied for many diseases. At first, it was used to evaluate the atherosclerotic plaque in cardiac coronary arteries and carotid arteries [8,9]. However, we delineated the focus of the deformity in cranial nerves (such as the trigeminal and facial nerves) in neurovascular compression syndrome, and that of the trochlear nerve, which was difficult to determine because it was very thin [10,11]. MSDE is useful for detecting brain metastases in tumor diseases [6]. 3D-CE-MSDE was reported to be useful in a case report of a patient with primary malignant glioma with broad spread to brain parenchyma [7]. However, no systematic research has been performed regarding primary malignant brain tumors in contrastenhanced MSDE. |
| Primary malignant brain tumors are characteristic of infiltrative invasion around brain parenchyma and disseminations in the subarachnoid space. Even if the tumor is completely surgically resected, invisible microinvasion of the tumor is common [1,2]. Therefore, tumor recurrence is also much more frequent in primary malignant brain tumors than in other malignant tumors, making the prognosis very poor even if chemoradiation therapy is performed after surgery [12,13]. The tumor resection rate during the operation has a significant influence on the prognosis [14]. In the evaluation of brain tumor image analysis, it is important to perform MRI sequences with gadolinium-based contrast media. To date, we have used conventional spin echo T1 weighted image and/or 3D-CE-T1TFE. SE-CE-T1WI has higher T1 contrast compared to 3D-CE-T1TFE. The contrast enhancement of 3D-CET1TFE has a tendency to be lower compared with that of SE-CET1WI, especially with a low concentration of gadolinium-based contrast media (0.1 mmol/L) [3]. Unfortunately, conventional SE-CE-T1WI is two-dimensional (2D) imaging, not 3D. SE-CE-T1WI does not allow observation of the brain tumor from all angles as do 3D-CE-T1TFE and 3D-CE-MSDE [15]. Therefore, it is difficult to detect small lesions such as disseminations using only SE-CET1WI. |
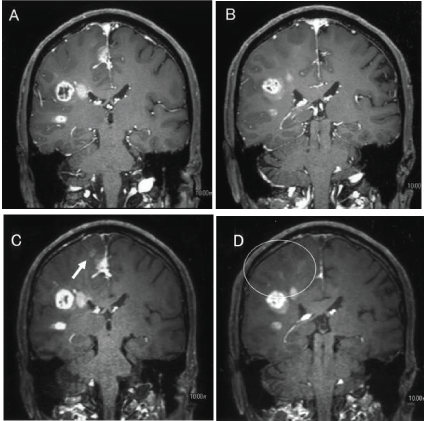
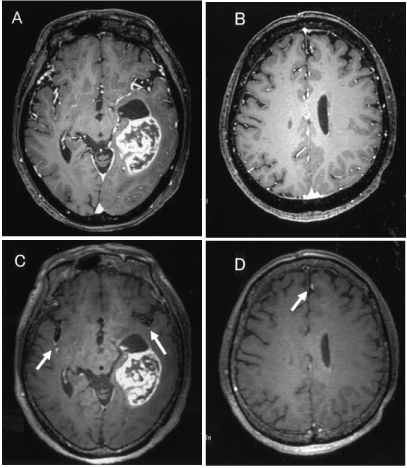
| 3D-CE-MSDE can suppress any flow signals such as cerebrospinal fluid and medullary veins and it can only delineate abnormal enhancement in brain parenchyma [4-11]. We showed that the CNR of 3D-CE-MSDE is significantly higher than that of 3D-CET1TFE in this study. It may reflect a large amount of enhancement in 3D-CE-MSDE images. As the time after injection of contrast media is longer, contrast enhancement is clearer [16]. However, we took into consideration the time after injection of contrast media in this study (see materials and methods). On the other hand, both radiologists separately determined that 3D-CEMSDE was superior to 3D-CE-T1TFE for detecting both intraaxial abnormal enhancement and disseminations (Figure 3A-D). Intraaxial abnormal enhancement may reveal blood brain barrier disorders that are a result of tumor invasion. It is important to accurately determine intraaxial abnormal enhancement in the preoperative study. 3D-CE-MSDE more clearly delineated the intraaxial abnormal enhancement compared with 3D-CET1TFE. Identifying disseminations is also easy using 3D-CE-MSDE because it can suppress intravessels’ enhancement (Figure 4AD). Thus, we may be able to identify disseminations without mistaking them for intravessels’ enhancement. We suggest that 3D-CE-MSDE is useful for detecting disseminations and intraaxial enhanced lesions. 3D-CE-MSDE may have influence on the surgical planning, targeting of radiation therapy and so on. |
| There are several limitations in this study. We have a small number of patients because primary malignant brain tumors are relatively rare. Further research involving a larger sample size of patients with primary malignant brain tumors is required. A biopsy for satellite intraaxial enhanced lesions and disseminations was not performed, so there is no pathological evidence for these enhanced lesions. Another limitation is that there was a slight discrepancy in the detectability of disseminations between the two radiologists. We did not prove malignant cells in the cerebrospinal fluid because of patients’ severe condition and actually several disseminated lesions did not change in the follow up study. This may be a result of the radiologists mistaking the enhanced vessel walls for disseminations. Thus, we need pay attention to distinguish disseminations from vessels wall enhancement. MSDE cannot completely suppress the enhanced vessel walls. Future research will involve clearly suppressing enhancement of the vessel walls and improving the quality of 3D-CE-MSDE images. |
Conclusions
|
| We tried to apply to 3D-CE-MSDE for making a diagnosis the primary malignant brain tumors. 3D-CE-MSDE is useful in detecting intraaxial enhanced lesions and disseminations for primary malignant brain tumors compared with the conventional sequences because it completely suppresses flow signals and has a high CNR. |
Acknowledgements
|
| This work was supported by a Grant-in-Aid from the Global COE program of the Japan Society for the Promotion of Science. All the authors have read the manuscript and have approved this submission. Obara M is a Philips employee and the other authors report no conflicts of interest. |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|









