Key words
|
| |
| Fast Dissolving Tablets, Gliclazide, Superdisintegrants, Direct Compression. |
| |
INTRODUCTION
|
| |
| A large number of patients may have difficulty in swallowing the conventional pharmaceutical dosage forms, particularly pediatric and geriatric. Such problems can be overcome by means of fast disintegrating / dissolving tablets. Fast disintegrating tablets are suitable for these patients since they immediately release the active drug when they are placed on the tongue. The fast-disintegrating tablets could be prepared using various techniques such as tablet molding, spray drying, sublimation, lyophilization, solid deposition or addition of disintegrants.(1) Recent advances in novel drug delivery systems aim to enhance safety and efficacy of drug molecules by formulating a convenient dosage form for administration and to achieve better patient compliance. One such approach led to development of fast dissolving tablets. (2-4) Advantages of this drug delivery system include administration without water, convenience of administration and accurate dosing as compare to liquids, easy portability, ability to provide advantages of liquid medication in the form of solid preparation, ideal for paediatric and geriatric patients and rapid dissolution/absorption of the drug, which may produce rapid onset of action. Some drugs are absorbed from mouth as the saliva passes down in to stomach and in such cases bioavailability of drug is increased, pre-gastric absorption can result in improved bioavailability and as result of reduced dosage form, improved clinical performance through a reduction of unwanted effects. Gliclazide is a second generation sulphonylurea oral hypoglycemic agent used in the treatment of non-insulin dependent diabetes mellitus. But the problem with this potentially useful hypoglycemic agent is that it is practically insoluble in water. This limits its oral bioavailability with large individual variation. After oral administration it get extensively metabolised by hydroxylation, N-oxidation and oxidation to several inactive metabolites. It is slightly soluble in water having half life 6-8 hrs. The drug is neutral in nature, molecule weight 323.4, melting point about 181°C and partition coefficient 2.1.(5) |
| |
Material and Methods
|
| |
|
Materials
|
| |
| Gliclazide was received as a gift sample from Cipla Pharmaceutical Ltd. (Pune, India), cross carmellose sodium and Microcrystalline cellulose was obtained as a free sample from Mapel biotech India Pvt. Ltd. Pune, cross povidone was obtained as a free sample from Zydus cadilla Ahmedabad, Dicalcium phosphate, Polyvinyl pyrolidone K-30, Pregelatinised starch, Aerosol, Magnesium Stearate were purchased from Fine chemicals. All other ingredients were of analytical grade. |
| |
|
Methods
|
| |
|
Formulation of fast dissolving tablets by direct compression method (6)
|
| |
| All the ingredients were weighed and passed through #60 mesh separately. Then the ingredients were mixed and compressed in to tablet using 6.5mm flat-faced punches on 10 station rotary tablet machine (Karnavati enterprises, Gujrat) The blend was compressed into tablets. Formulations of Gliclazide Fast dissolving tablets by direct compression method are shown in Table 1. |
| |
|
Evaluation parameters of fast dissolving tablets: Hardness (7)
|
| |
| The tablet hardness, which is the force required to break a tablet in a diametric compression force. The hardness tester used in the study was Monsanto hardness tester, which applies force to the tablet diametrically with the help of an in built spring. |
| |
|
Friability (7)
|
| |
| The friability of a sample of 20 tablets was measured using Roche Friabilator (Veego Lab. Mumbai, India). Twenty tablets were weighed, rotated at 25 rpm for 4 minutes. Tablets were reweighed after removal of fines and the percentage of weight loss was calculated. Friability below 1% was considered acceptable. |
| |
|
Weight variation test (7)
|
| |
| Weight variation test was done by weighing 20 tablets individually, calculating the average weight and comparing the individual tablet weight to the average weight. |
| |
|
In vitro disintegration time (7)
|
| |
| The disintegration time of the tablet was measured using Digital Disintegration test apparatus ( Veego Lab. Mumbai, India) in water (37± 2°C) according to disintegration test apparatus with disk. The time in seconds taken for the complete disintegration of the tablet with no palpable mass in the apparatus was measured in seconds. Three tablets from each batch formulation were tested for the disintegration time calculations. |
| |
|
Wetting time ( 8)
|
| |
| A piece of tissue paper folded twice was placed in a small petri dish (ID= 6.5 cm) containing 6 ml of simulated saliva pH 6.8, a tablet was put on the paper, and the time for complete wetting was measured. |
| |
|
In vitro dissolution profile (9)
|
| |
| Dissolution studies were carried out by USP paddle method Type II apparatus at 37± 0.50 ° c, taking 900 ml of phosphate buffer pH 6.8 as a dissolution medium. Speed of rotation of paddle was set at 50 rpm. Absorbance of sample was measured at 226 nm by using UV spectrophotometer. |
| |
|
Stability studies (10)
|
| |
| Stability studies were carried out at 25°c / 60% RH and 40°c/ 75% RH for 60 days for optimized formulation GF8 according to ICH guidelines. |
| |
RESULT AND DISCUSSION
|
| |
| The present investigation was undertaken to formulate and evaluate fast dissolving tablets of gliclazide by direct compression method using Croscarmellose sodium and crospovidone as a superdisintegrants. Superdisintegrants are generally used by formulation scientists for developing Fast dissolving tablets or for improvement of solubility for drugs. The primary requirement for both dosage forms is quicker disintegration. The amount of Superdisintegrants was optimized in the formulation of FDTs. The total 9 were formulation (GF1-GF9) prepared using different concentration of Croscarmellose sodium and crospovidone to study its effect on disintegration time. The results for evaluation of different batches of Gliclazide formulation by direct compression method are shown in Table 2. |
| |
| Percent weight variation was observed between 4.0 and 6.1 which were well within the acceptable limit for uncoated tablets as per United States Pharmacopoeia. It is well known to formulation scientists that the tablets with more hardness show longer disintegration time. Since mechanical integrity is of paramount importance in successful formulations, hence the hardness of tablets was determined and was found to be in the range of 3.4 to 3.8 Kg/cm2. Friability was observed between 0.40 and 0.59 %, which were below 1% indicating sufficient mechanical integrity and strength of prepared tablets. The disintegration time for all formulations was found to be 12-22 seconds and wetting time was 17- 43 seconds. The In vitro dissolution study was performed for all formulations and the results are shown in Table 3. |
| |
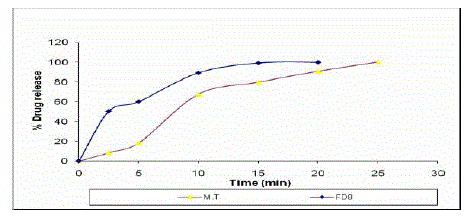
| In vitro dissolution studies showed that more than 50% of the drug was released from the all formulations within 5 minutes. The GF8 formulation containing croscarmellose sodium in concentration of 5% showed minimum disintegration time of 12.42 seconds, wetting time of 17.50 seconds and 51.55% drug and 99.79% drug was released within 3 minutes and 24 minutes respectively. The optimized formulation of FD8 was compared with marketed tablet (Glucotrol 5mg) and the dissolution parameters of both formulations are shown in Table 4 and Fig 1. |
| |
| From the dissolution studies, it was confirmed that the more than 99% drug release for optimized formulation was within 12 minutes, where as the marketed tablet showed the 99.86% at 24 minutes. |
| |
| Stability studies for optimized formulation GF8 was carried out at 250°c/ 60% RH and at 40 °c/ 75% RH and the results are shown in Table 5. |
| |
CONCLUSION
|
| |
| Fast dissolving tablets of Gliclazide were prepared by direct compression method using Croscarmellose sodium and crospovidone as a superdisintegrants. The tablets disintegrated rapidly in oral cavity and had acceptable hardness and friability. In vitro drug release from the tablets shows significantly improved drug dissolution. It was concluded that in direct compression method, croscarmellose sodium was best superdisintegrant with pvpk- 30 as binding agent. Hence it could be concluded that the superdisintegrant based fast dissolving tablets of Gliclazide would providing quick onset of action without need of water for swallowing or administration. Further investigations are needed to confirm the in vivo efficiency |
| |
ACKNOWLEDGEMENTS
|
| |
| Authors are wish to acknowledge Cipla Pharmaceutical Ltd. (Pune, India), for providing Gliclazide as gift sample. Authors are also grateful to Mapel biotech India Pvt. Ltd for providing, crosscarmellose sodium and microcrystalline cellulose as gift samples and the principal Dr. H. N. More Bharati Vidyapeeth College of Pharmacy Kolhapur for providing excellent facility to carry out this work. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
| Figure 1 |
|
| |







