Solanki Sagar D*1, dr. Patel Paresh U2 and Dr.Suhagiya Bhanubhai N3
1K.B.Raval college of pharmacy., Sertha
2S.K.Patel college of pharmacy., Kherva
3L.M.College of pharmacy., Ahmedabad.
- Corresponding Author:
- Mr. Sagar D. Solanki
K.B.Raval college of pharmacy
B/h Kasturinagar,At & Po Sertha Dist- Gandhinagar.
Email: sagarrx@yahoo.co.in
Date of Submission: 18-07-2011 Date of Acceptance: 12-09-2011
Citation:Solanki Sagar D, Dr. Patel Paresh U, Ajmera Ankit A, Patel Jignesh C, Talaviya Smita M, Parmar Parul K “Development And Validation Of Reversed-Phase High Performance Liquid Chromatographic Method For Estimation Of Sumatriptan Succinate In Pharmaceutical Dosage Form”,Int. J. Drug Dev. & Res., July-Sep 2011, 3(3): 266-269
Copyright:© 2010 IJDDR, D Sagar Solanki et al. This is an open access paper distributed under the copyright agreement with Serials Publication, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords
Sumatriptan succinate, HPLC.
Introduction
Sumatriptan succinate (SUMA) is chemically 3-[2-(dimethylamino)ethyl]-N-methyl- indole-5-methanesulfonamide succinate (Figure :1). Sumatriptan succinate is official in British pharmacopoeia[1], European Pharmacopoeia [2] and United States Pharmacopoeia [3]. It is a selective 5- hydroxytryptamine receptor subtype agonist and used as antimigraine drug. SUMA is a selective agonist of vascular serotonin ((5-hydroxytryptamine; 5-HT) type 1-like receptors, likely the 5-HT1D and 5- HT1B subtypes[4].
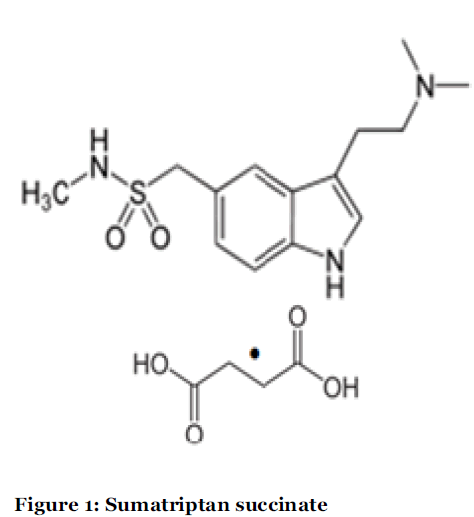
Figure 1: Sumatriptan succinate
A literature survey regarding quantitative analysis of SUMA revealed that attempts were made to develop analytical methods for SUMA in combination with ergotamine tartrate by HPLC [5] sumatriptan by HPTLC[6] and visible spectrophotometry[7]. The aim of our investigation was to develop and validate an LC method for the determination of SUMA in pharmaceutical dosage forms.
In this study, reversed-phase high performance liquid chromatographic method has been developed for determination of Sumatriptan succinate and applied to commercial tablet dosage forms. The results obtained were validated according to the ICH guidelines.
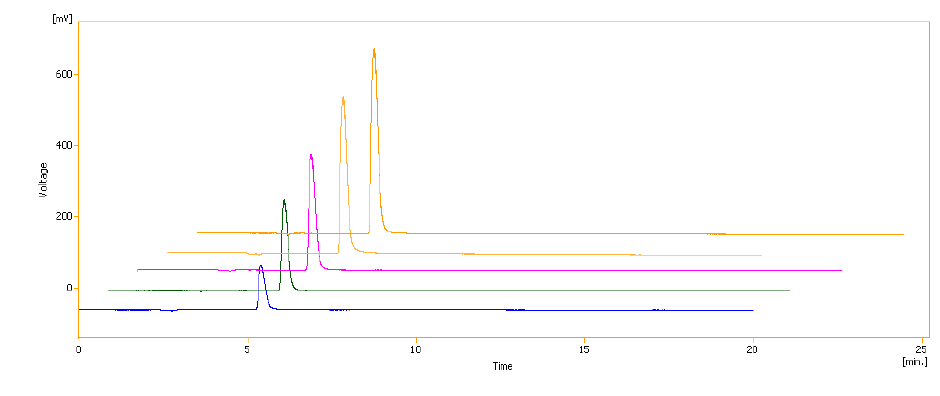
Figure 2: Chromatogram of Sumatriptan succinate.
2. Materials and Methods
2.1. Chromatographic conditions
The HPLC system consisted of a Young Lin 9101 vaccum degasser, a Young Lin 9001 quaternary pump and a Young Lin 9160 PDA detector (Seoul, South Korea). An YL-clarity chromatography data system was used to record and evaluate the data collected during and following chromatographic analysis. The chromatographic separation was achieved on a Purospher® 5μm, 250mm X 4.6mm. The mobile phase consisting of ACN : Water (18:82) and 0.05% v/v trifluro acetic acid was added in water, pumped at a constant flow rate of 1.0 ml/min. The eluent was monitored using PDA detector at a wavelength of 232 nm.
The column was maintained at room temperature and injection volume of 20μl was used. The mobile phase was filtered through 0.45μm Chrom Tech Nylon-66 filter to use.
Ultra sonic cleaner (Life care equipment pvt. Ltd.)
2.2 Reagents
Sumatriptan succinate was kindly provided as gift sample by Astron Pharmaceuticals, Ahmedabad. Commercially available Suminat 50 tablets (Sun pharmaceutical) were purchased from local market, India.
Acetonitrile (ACN) and water [HPLC grade] were purchased from the Merck [INDIA].
2.3 Preparation of standard solution and calibration graphs
The stock solutions of SUMA was prepared by dissolving accurately weighed 25 mg of the drug, transferred to 25 ml volumetric flask, dissolved and made up to the volume by using water (HPLC grade). Then appropriate dilutions were made to adjust the final concentration 10, 20, 30, 40, 50 ppm. The results of calibration curve are shown in table 1.
| S. no. |
Parameters |
SUMA |
| 1 |
Linear Range (mg/ml) |
10-50 µg/ml |
| 2 |
Retention time (min) |
5.20 |
| 3 |
Slope |
130.87 |
| 4 |
Intercept |
418.7 |
| 5 |
Linear equation |
y = 130.87x +418.7 |
| 6 |
R2 value |
0.9974 |
Table 1. Data from standard curve of SUMA by RPHPLC method.
2.4.Preparation of sample solution
For the estimation of drugs in Suminat 50 tablets, twenty tablets were accurately weighed, crushed and powdered in a glass mortar. The tablet powder equivalent to 50 mg SUMA was transferred accurately to a 50 ml volumetric flask and diluted to volume with water (HPLC grade). The solution was further diluted to obtain concentration of 40 ppm and the results are shown in table 2.
S.
no. |
Formulations |
Actual concentration
(mg/ml) |
%SU MA |
| 1 |
Suminat 50 |
40(mg/ml) |
101.92 |
| |
(Sunpharmaceu |
|
% |
| |
tical) |
|
|
Table 2. Assay Results of Market Formulation
2.5 Method validation [8]
The developed methods were validated for parameters like accuracy, precision, linearity and range, LOD, LOQ, ruggedness and specificity etc, according to the ICH guidelines. The data for which are presented in the table 3.
2.5.1 Accuracy
Accuracy was determined by adding the three different quantities [Low, Medium, and High] of the standard sample to the sample solution containing the concentration of 10 μg/ml. The results are shown in table 3.
S.
no. |
Parameters |
Sumatriptan |
| 1 |
Recovery (%) |
98.55 - 101.86 |
| 2 |
Repeatability (RSD, n=6) |
0.7025- 1.1928 |
| 3 |
Precision Range(CV) |
|
| 4 |
Intra-day (n=3) |
0.5816- 1.1588 |
| 5 |
Inter-day (n=3) |
1.0124- 1.6543 |
| 6 |
Limit of Detection (µg/ml) |
0.90 |
| 7 |
Limit of Quantification
(µg/ml) |
2.72 |
Table 3. Summary of Validation Parameter
2.5.2 Repetability
Repeatability was determined on 6 replicate of each concentration of the standard solution. The results are shown in table 3.
2.5.3 Precision
Precision was determined by performing Intra day and Inter day determination concentration on three different concentration as shown in table 3.
2.5.4 Limit of Detection and Limit of Quantification
The limit of Detection (LOD) and limit of Quantification (LOQ) were determined according to the ICH guidelines.
Where detection limit DL = 3.3σ and quantitation limit QL = 10σ S
Where σ = standard deviation of y-intercepts of regression lines
S = the slope of the calibration curve
The result is shown in table 3.
2.5.5 Ruggedness
The ruggedness of the method was determined by carrying out the experiment on different instruments. The %RSD values with two different instruments were 0.70-1.05 and 0.63-1.44 respectively.
3. Results and Discussions
The reversed-phase LC method described in this paper was developed for determination of SUMA in tablet dosage form. The method was validated according to ICH guidelines.
The linearity of the peak response versus concentration was studied from 10 to 50 μg/mL. The representative linear equation was y = 130.87x +418.7 and correlation coefficient (r) is 0.9974.
Recovery study was performed in triplicate and was found in range of 98.55 - 101.86. The precision is usually expressed as the %RSD and it was found to be 0.7025- 1.1928. The Inter-day and intra-day precision were 1.0124- 1.6543 and 0.5816- 1.1588 respectively.
An economic, simple and rapid RP-LC method has been developed for determination of SUMA in tablet dosage forms. The proposed method is simple, accurate and precise for the quantification of SUMA in tablet dosage form as well as bulk drugs for routine analysis.
Conflict of Interest: NIL
Source of Support: NONE
5654
References
- United States Pharmacopoeia and National Formulary; (24th) Asian Edition,
- The United States Pharmacopoeia Convention Inc, U.SA, page no. 2709-3259.
- Havaldar F H* and Vairal D L, International journal of chemical and analytical science2010, Volume 1, No 4.
- Shah C R*, Suhagia B N, Shah N J, and Shah R R, Indian journal of Pharmaceutical Sciences 2008 Nov-Dec; 70(6): 831–834.
- Buridi.kalyana ramu* and K. Raghubabu, Internation journal of applied biology and pharmaceutical technology 2011, Volume: 2: Issue-1.
- Validation of Analytical Procedures; Text and Methodology (Q2B), ICH Harmonised Tripartite Guideline.








