Key words
|
| |
| Anti-inflammatory, Antimicrobial, Indomethacin, Nystatin, Microorganism |
| |
INTRODUCTION
|
| |
| Itraconazole is a potent triazole antifungal agent that is prescribed to patients with fungal infections used for the treatment of mycoses. The drug may be given orally or intravenously.1-3 The IUPAC nomenclature of the drug is as follows: (2R,4S)-rel-1- (butan-2-yl)-4-{4-[4-(4-{[(2R,4S)-2-(2,4- dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-ioxolan-4-yl]-methoxy}-phenyl)-piperazin yl]phenyl}-4,5-dihydro-1H-1,2,4-triazol-5-one. |
| |
| ITZ is used orally in the form of capsules for treatment of dermatophyte infections, occurrence of superficial fungal infections and systemic fungal infections. For quality control and stability testing of Itraconazole in pharmaceutical formulations, limited methods have been published, because the drug is not yet official in any pharmacopoeia. Spectrofluorimetry method has been used for assay of Itraconazole in raw material and in dosage forms. RP-HPLC method is used for determination of Itraconazole in human plasma. 4-7 Chromatographic separation in this method was performed on an octadecylsilane column using fluorescence detector. However, it has the disadvantage of being time consuming. All these studies have furth emphasized the need to perform rapid and sensitive quality-control analysis of pharmaceutical formulations containing Itraconazole. As these methods are expensive, we have made an attempt to develop a more precise, simple and economical spectrophotometric method with greater precision, accuracy and sensitivity for the analysis of Itraconazole in bulk and dosage forms. |
| |
|
Experimental
|
| |
|
Chemicals and reagents
|
| |
| Methanol was used throughout UV spectrophotometric method development and validation. |
| |
|
Instrumentation
|
| |
| UV spectrophotometric method was performed on double beam UV-visible spectrophotometer (Shimadzu, model 1700) having two matched quartz cells with 1 cm light path. |
| |
|
Selection of solvent
|
| |
| Methanol was selected as ideal solvent for spectrophotometric analysis of Itraconazole. |
| |
|
Preparation of standard stock solution (1000μg/ml)
|
| |
| Accurately weighed quantity of 100 mg Itraconazole reference standard was transferred into 100 ml volumetric flask and dissolved and diluted up to the mark with methanol to give a stock solution having strength 1000μg/ml. 100 |
| |
|
Preparation of sample stock solution (100μg/ml)
|
| |
| To measure the ITZ content of capsule (label claim 100 mg ITZ per capsule, Itaspor capsules), twenty capsules were weighed, the mean weight was determined. A weight of the powder equivalent to 100 mg ITZ was transferred to a 100 ml volumetric flask containing 50 ml methanol and the mixture was sonicated for 30 min then diluted to 100 ml with methanol (1000 μg/ml). The solution was filtered and 1 ml of filtered solution was diluted tenfold to furnish a concentration of 100μg/ml. |
| |
|
Validation of UV spectrophotometric method Linearity and range
|
| |
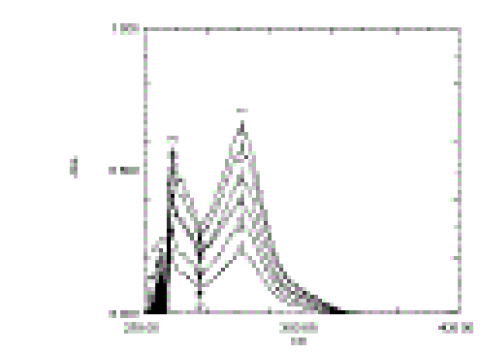
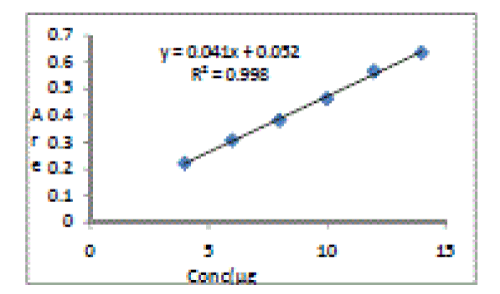
| The linearity was determined by analyzing 6 independent levels of calibration curve in the range of 4-14μg/ml. Absorbance of each solution against methanol was recorded at 262 nm. The calibration curve of absorbance vs. concentration was plotted and correlation co-efficient and regression line equation for Itraconazole were determined. |
| |
|
Precision
|
| |
| Intra-day precision was determined by analyzing Itraconazole (4-14 |
| |
|
Accuracy
|
| |
| Accuracy was determined by performing recovery studies by spiking different concentrations of pure drug in the pre-analyzed powder for infusion samples within the analytical concentration range of the proposed method at three different set at level of 80%, 100% and 120%. The amount of Itraconazole was calculated at each level and % recoveries were computed. |
| |
|
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
|
| |
| The LOD and LOQ were estimated from the set of 5 calibration curves used to determine method linearity. |
| |
| LOD= 3.3*σ/S and LOQ= 10*σ/S |
| |
| Where, σ = the standard deviation of y-intercepts of regression lines |
| |
| S = the slope of the calibration curve |
| |
|
Analysis of marketed formulation (Itaspor capsule) by UV spectrophotometric method
|
| |
| From the sample stock solution, 1 ml was taken in 10 ml volumetric flask and diluted up to the mark with distilled water. (10 μg/ml) |
| |
Result and discussion
|
| |
|
Linearity
|
| |
| The linearity of Itraconazole was found to be in the range of 4-14 μg/ml with correlation co-efficient 0.9988. Calibration data with %RSD is shown in table 1 and calibration curve is shown in Figure 2. |
| |
|
Accuracy
|
| |
| Accuracy of the method was confirmed by recovery study from marketed formulation at three level of standard addition. % Recovery for Itraconazole was found to be 99.11-101.18. |
| |
|
Limit of Detection and Limit of Quantitation
|
| |
| LOD and LOQ were found to be 0.146 |
| |
|
Analysis of marketed formulation (Itaspor capsule) by UV spectrophotometric method
|
| |
| The percentage of Itraconazole in marketed formulation (Itaspor capsule) was calculated from the calibration curve of Itraconazole. %Assay was found to be 100.44% as shown in Table 4. |
| |
RESULTS AND DISCUSSION
|
| |
| The proposed method for the determination of Itraconazole in solid dosage form was found to be precise, selective, rapid and economical. Itraconazole exhibited maximum absorption at 262 nm and obeyed Beer’s law in the concentration range of 4-14 2) of 0.9982 (Figure 3). A relative standard deviation of 0.66 % was observed on analysis of six replicate samples. Our studies revealed a recovery percentage of 99.11- 101.18%, which indicates that the developed method was simple, rapid and precise. The proposed methods can be used for the drug analysis in routine quality control & method proves to be more economical than the published standard methods. |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |