Research - (2022) Volume 13, Issue 4
Development of bioresorbable braided self expanding implantable neurovascular flow diverter for intracranial aneurysm
Pramod Kumar,
Kothwala Deveshkumar Mahendralal* and
Durani Mohamadovesh Mohamadyasin
Meril Life Sciences Pvt. Ltd, Bilakhia House, Muktanand Marg Chala, Vapi, Valsad, 396 191, Gujarat, India
*Correspondence:
Kothwala Deveshkumar Mahendralal, Meril Life Sciences Pvt. Ltd, Bilakhia House, Muktanand Marg Chala, Vapi, Valsad, 396 191,
Gujarat,
India,
Email:
Received: 30-Mar-2022, Manuscript No. ipjnn-22-12698;
Editor assigned: 02-Apr-2022, Pre QC No. P-12698;
Reviewed: 19-Apr-2022, QC No. Q-12698;
Revised: 23-Apr-2022, Manuscript No. R-12698;
Published:
30-Apr-2022
Abstract
An intracranial aneurysm can be treated with an implantable self- expanding ultrathin bioresorbable neurovascular device. Inside the intracranial aneurysm, the braided self-expanding bioresorbable neurovascular implanted device obstructs the blood flow to enter inside an aneurysm. The biocompatible and totally bioresorbable ultrathin braided mesh structure with a specified braiding arrangement and reduced pore size that redirects blood flow to limit blood flow inside aneurysm sac. A braided neurovascular implant with an elastomeric compound coating provides sufficient radial strength, axial flexibility, and excellent self-expanding capabilities. The self-expandable bioresorbable braided scaffold retains structural integrity for about a year before resorbing over a two- to three-year time frame
Keywords
Self expanding bioresorbable; Neurovascular; Aneurysm.
Introduction
Aneurysms can be caused by a number of factors, including
high blood pressure and atherosclerosis, trauma, genetics,
and irregular blood flow. A gigantic aneurysm can be more
than 2.5 centimetres wide and include more than one artery.
High blood pressure (hypertension) causes damage and
weakening of blood vessels over time [1]. The formation of
fatty plaques (atherosclerosis) causes a weakening of the blood
vessel wall. Inherited illnesses that cause blood vessel walls to
be weaker than usual. A gigantic aneurysm can be more than
2.5 centimetres wide and include more than one artery [2].
The anterior (carotid) circulation accounts for approximately
86.5 percent of all cerebral Aneurysms. An infected arterial
wall causes a mycotic aneurysism - an abnormal bulge on the
inside of an artery. For more than 40 years, surgical clipping
has been performed to treat cerebral aneurysms. Aneurysm
clipping and endovascular procedures like as coiling, stentassisted
coiling, and flow diversion stents are two of the most
common treatment choices. A ruptured cerebral aneurysm
prognosis is determined by the aneurysm size and location, as
well as the patient age, health, and neurological status [3,4].
An aneurysm is a ballooning at a weak spot in an artery wall.
An aneurysm walls can be thin enough to rupture. Early
bleeding from a burst brain aneurysm can kill some people.
A bad outcome, death or lifelong impairment affects around
two third of patients. It is an endovascular procedure that
entails introducing a micro catheter into the femoral artery.
Instead of introducing a device inside the aneurysm sac, as
with coiling, a device is placed in the main blood vessel to
divert flow of blood away from the aneurysm. In present
treatment, the use of a self-expanding non degradable flow
diverter has drawbacks such as corrosion and toxicity in the
implant location. Present work also pertains to a process for
producing a bioresorbable flow diverter for neurovascular
implants that has high strength, great flexibility, and a small
pore size [5]. This is more especially connected to employing
PLLA or PLGA material to fabricate tubular braid devices.
The braided construction with smaller pore sizes helps to
divert the blood flow and prevent blood from penetrating
the aneurysm sac. The treatment of intracranial aneurysms
with devices by covering the aneurysm neck. Mechanisms of
the delayed rupture are actually not completely elucidated.
Very late thrombosis of the flow diverter is possible and
long term follow up of treated patients is certainly required.
Combinations of the bioresorbable filaments can also be
used to make braided tubes. Another potential mechanism involves intra aneurysm thrombosis created by flow diversion
which can be associated with an inflammatory reaction.
Materials and Methods
The micro porous bioresorbable braided tube with open
and closed angle provides high strength, flexibility and
a small pore size. The groups of several shape memory
polymers such as Poly L- lactide-co-caprolactone (PLC),
Poly caprolactone (PCL), Poly - dl -lactic acid (PDLLA),
Poly glycerol Sebacate (PGS), Poly L-lactide (PLLA),
Poly glycolic Acid (PGA), Poly L- lactide co- glycolic acid
(PLGA) or mixture therefore used in the braiding process.
The extruded monofilament is annealed for allowing it to
endure high tension braiding. The implant is coated with
bioresorbable elastomer [6]. Heat treatment was carried
out under vacuum conditions. An anti-thrombogenic,
anti-inflammatory, or any specific hormonal drug is
coated on an elastomers coated flow diverter device. The
microporous braided bioresorbable implant was made with
20-50 micron monofilaments and a 32-96 carrier braiding
machine. Each implant has a porosity of about 24 to 120.
Many filaments are braided together over a mandrel with
braiding angles ranging from 30°C to 200°C. The pores
formed by the braiding pattern span from 12 to 30 pores
per millimeter [7-9]. The flow diverter braiding angle is
inversely proportional to the pore size. PET mandrels are
held in a controlled vacuum and annealed at a temperature
ranging from 90°C to 130°C, for a period of time ranging from 14 to 20 hours. The polymer braided tube is fixed
on a mandrel at both ends and then annealed to stabilize
the braided structures. Braided mandrels are kept under
vacuum in a controlled environment of 500-800 mm Hg
pressure. Annealing is a process in which the body is held at
a temperature between 90°C to 130°C for 12 to 20 hours.
The terminal annealing, on the other hand, is carried out
from 1 to 5 hours with a temperature of 90°C to 110°C.
The other forty six monofilaments are employed with the
two 20-50 micron platinum tungsten monofilaments are
used for each marker, with an elastomer coating thickness
ranging from 1 to 10 m. The internal diameter of the marker
is 35-45 microns, while the wall thickness is 2-5 microns
[10]. Each marker is 0.1-0.2 mm long or as specified by
the braided construction. A flow diverter with elastomeric
coating was packed in vacuum desiccators for 8-20 hours at
room temperature and then cured between 70°C to 140°C.
The radial strength of non-elastomers coated braided tube
ranged from 5 to 20 N, but increased from 20 to 30 N,
when it was braided with the coating. The distance between
the braided flow diverter and the spray gun should be kept
between 2 to 4 cm and rotation should be between 20 to
30 rpm, which is important in achieving a smooth and
uniform coating (Fig. 1-6).

Fig 1: Bioresorbable braided tube.
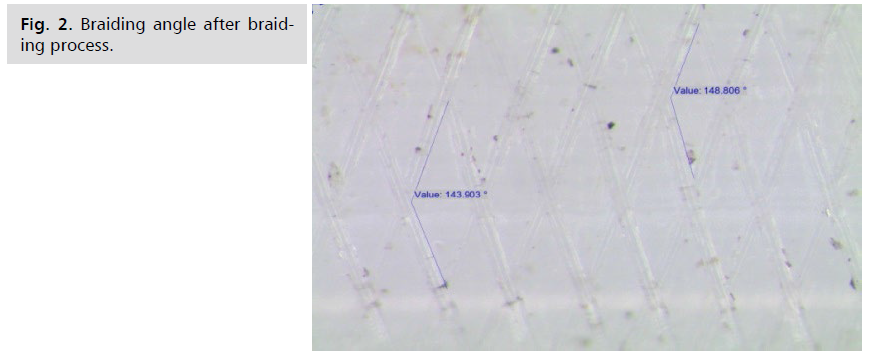
Fig 2: Braiding angle after braiding process.

Fig 3: Open cell at both side of flow diverter.
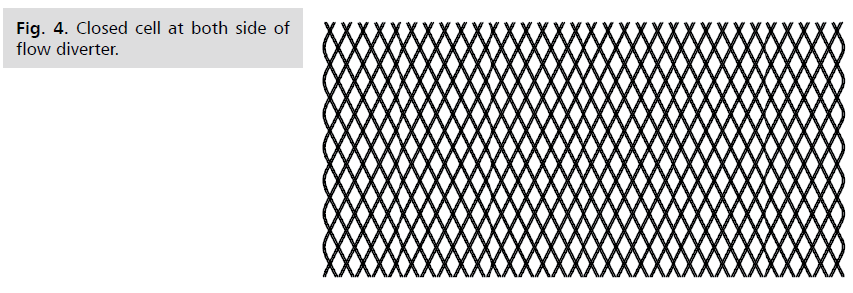
Fig 4: Closed cell at both side of flow diverter.
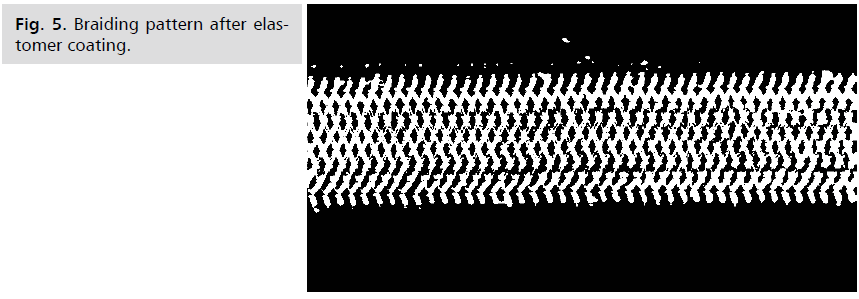
Fig 5: Braiding pattern after elastomer coating.
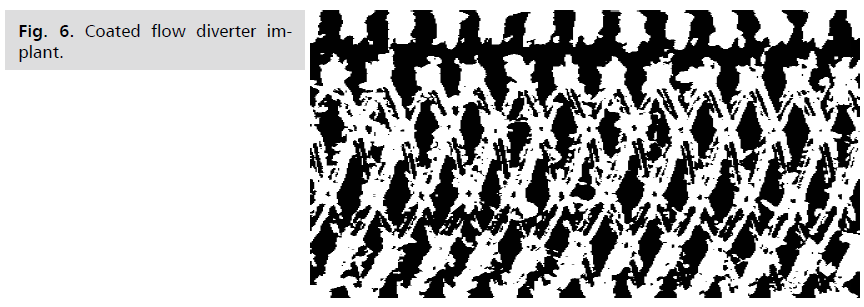
Fig 6: Coated flow diverter implant.
Results and Discussion
Intracranial aneurysm stenting is a procedure that involves
redirecting and rerouting the arterial blood flow and creating a way to avoid rupture of aneurysm to prevent
blood from entering the brain [11,12]. The microporous
braided implant incorporates small pores of bioresorbable
material with particular braiding structure, resulting in
sufficient radial strength, foreshortening, and other selfexpanding
stent properties. The initial annealing procedure
aids in the elimination of monomer and the relaxation of
internal stress in braided monofilament [13]. The flow
diverter braided mesh angle has a direct impact on its
pore size and as a result, radial stiffness which is a critical characteristic determining the integrity and structure
after being inserted into the body lumen. Annealing of
the braid cross wire by lowering the melting point of the
polymer increases the mechanical strength of the braided
arrangement and allows for a comparable configuration
following deployment in the artery lumen. The analytic
evidence presented here clearly demonstrates that the
micro porous structure maintains its properties throughout
the operation [14].
Sample results
Material characteristics of samples before and after
monofilament annealing are given in following Chart. 1-4.
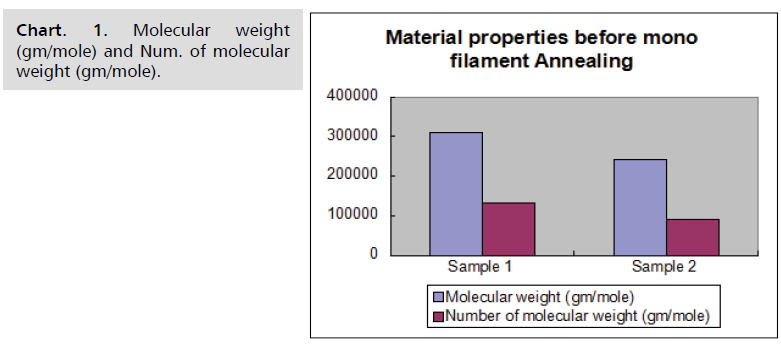
Chart. 1. Molecular weight (gm/mole) and Num. of molecular weight (gm/mole).
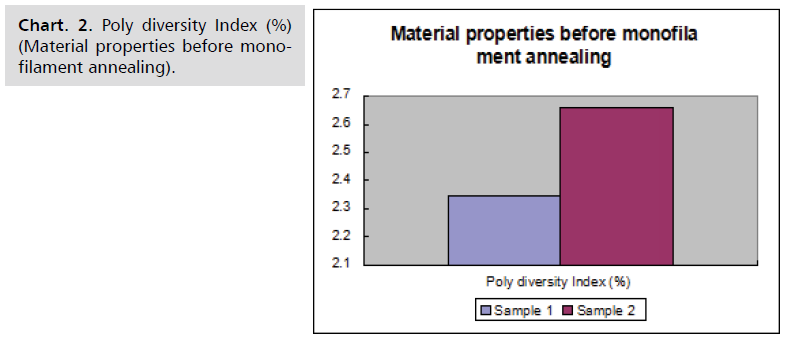
Chart. 2. Poly diversity Index (%) (Material properties before monofilament annealing).
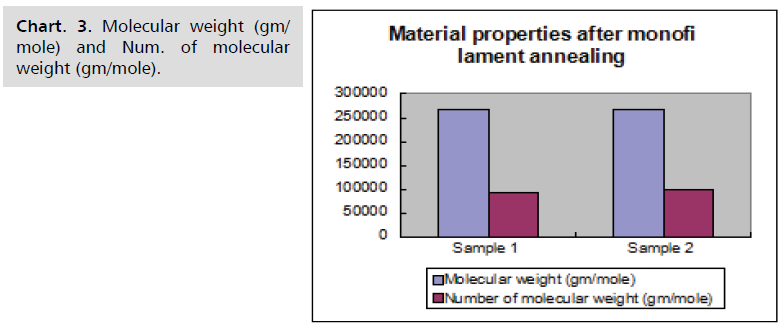
Chart. 3. Molecular weight (gm/mole) and Num. of molecular weight (gm/mole).
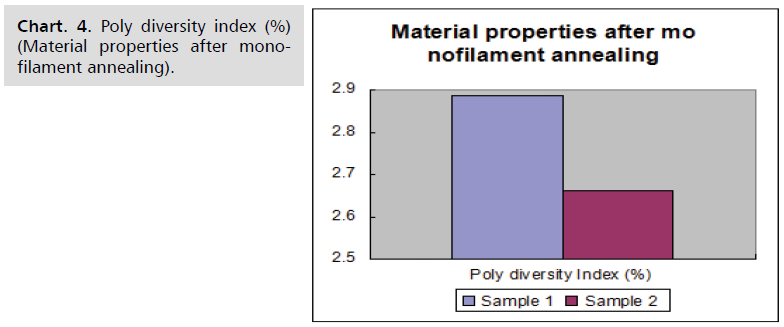
Chart. 4. Poly diversity index (%) (Material properties after monofilament annealing).
The material properties of BRS flow diverter without coating shown in below Tab. 1.
| Material Properties after Annealing of BRS Flow Diverter (without coating) |
| Sample Details |
Sample-1 |
Sample-2 |
| Molecular weight (gm/mole) |
377678 |
402250 |
| Number of molecular weight (gm/mole) |
160340 |
211278 |
| PDI (Polydispersity Index) |
2.355 |
1.904 |
| Glass transition temperature (°C) |
57.26 |
53.13 |
| Melting temperature (°C) |
187.36 |
187.46 |
| % Crystallinity |
64.30 |
68.50 |
| Radial strength of BRS flow diverter |
9.25 N, 9.17 N, 10.96 N |
Tab. 1. Material properties after annealing of BRS flow diverter (without coating).
The material properties of BRS flow diverter was coated
with elastomeric coating, which given the following results
(Tab. 2).
| Material Properties after Elastomeric Coating of BRS Flow Diverter |
| Sample Details |
Sample-1 |
Sample-2 |
| Molecular weight (gm/mole) |
345856 |
335633 |
| Number of molecular weight (gm/mole) |
145567 |
133464 |
| PDI (Polydispersity Index) |
2.376 |
2.515 |
| Glass transition temperature (°C) |
60.19 |
50.71 |
| Melting temperature (°C) |
187.35 |
187.16 |
| % Crystallinity |
62.30 |
63.40 |
| Radial strength of BRS flow diverter |
84.73 N, 87.90 N, 67.76 N |
Tab. 2. Material properties after elastomeric coating of BRS flow diverter.
Process sample results
The properties of polymer, such as molecular weight,
number of molecular weights, polydispersity index (PDI),
glass transition temperature, melting temperature, and
percent crystallinity, do not change when the polymer
is exposed to different process parameters - such as
temperature and time shows in the Chart. 5-8.
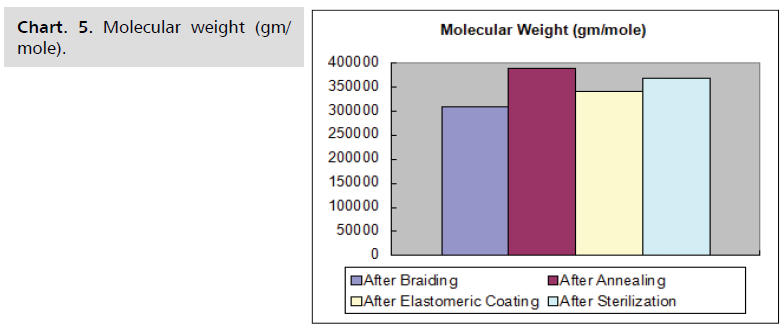
Chart. 5. Molecular weight (gm/mole).
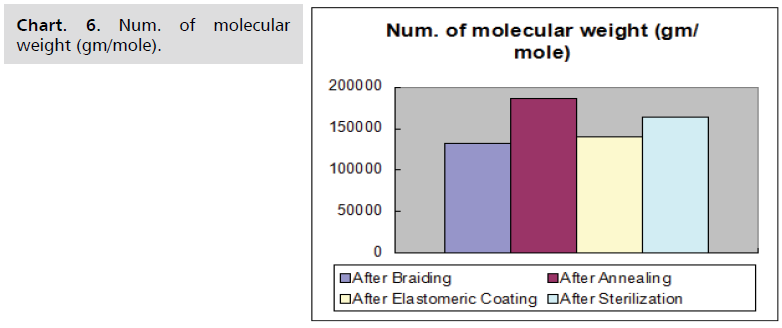
Chart. 6. Num. of molecular weight (gm/mole).
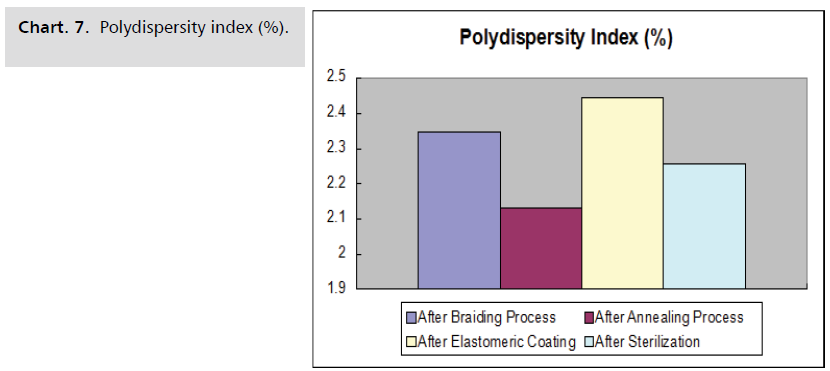
Chart. 7. Polydispersity index (%).
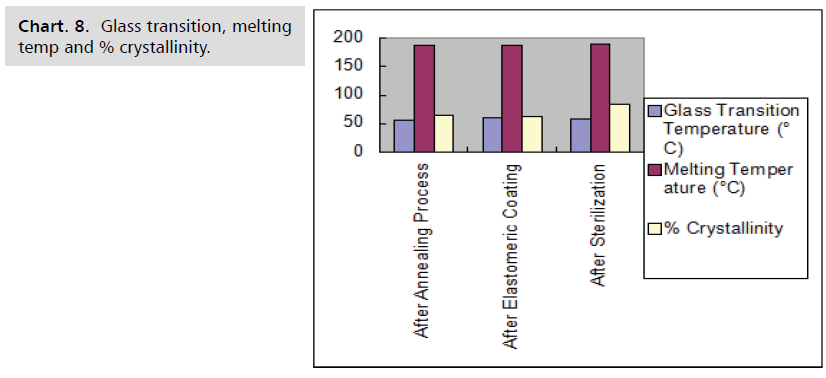
Chart. 8. Glass transition, melting temp and % crystallinity.
Degradation process result
The following Charts. 9-11 shows, when a flow diverter
is subjected to accelerated degradation, the parameters of
the polymer, such as glass transition temperature, melting
temperature, and % crystallinity, steadily decrease with
time.
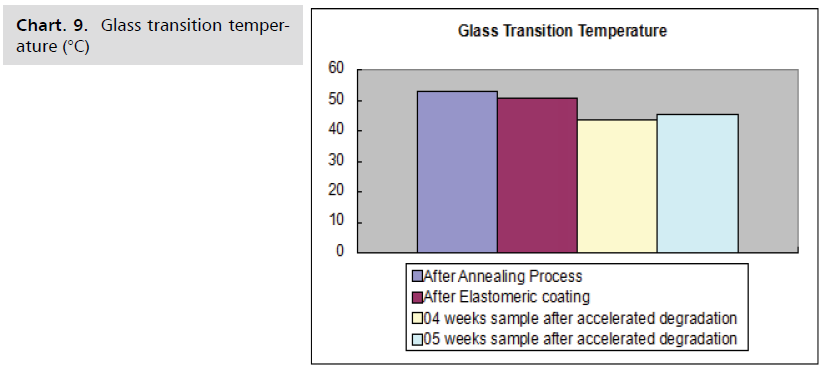
Chart. 9. Glass transition temperature (°C)
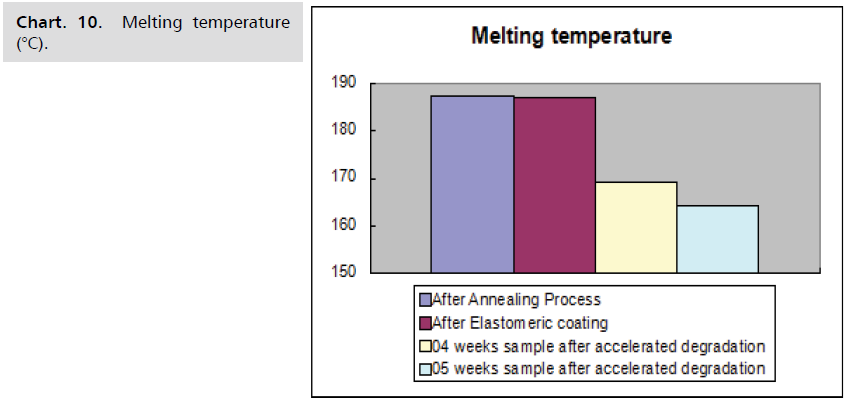
Chart. 10. Melting temperature (°C).

Chart. 11. % crystallinity.
New degradation data in in vitro condition
The following chart shows the accelerated in vitro
simulation study at 70°C interval days.
Conclusion
The treatment of intracranial aneurysms with flow
diverters seems to be highly efficacious. A bioresorbable
ultrathin braided mesh structure with a smaller pore size redirects blood flow to prevent blood from flowing into
an aneurysm. The safety of this treatment appears to be
satisfactory, specifically in the context of treating complex
aneurysm. Flow diverters have been proposed for use in
very small ruptured aneurysms that are untreatable using
standard endovascular techniques. Bioresorbable materials
are thought to be safer and more biocompatible. The
biodegradable solution could meet the short-term needs of
a sick patient while avoiding the long-term risks of dense
metal mesh.
REFERENCES
- Jamshidi M, Rajabian M, Avery MB, et al. A novel self-expanding primarily bioabsorbable braided flow-diverting stent for aneurysms: initial safety results. J Neuro Interv Surg. 2020; 12(7):700-705.
Google Scholar, Crossref, Indexed at
- Howe C, Mishra S, Kim YS, et al. Stretchable, implantable, nanostructured flow-diverter system for quantification of intra-aneurysmal hemodynamics. ACS Nano. 2018;12(8):8706-8716.
Google Scholar, Crossref, Indexed at
- Henkes H, Weber W. The past, present and future of endovascular aneurysm treatment. Clin Neuroradiol. 2015;25(2):317-324.
Google Scholar, Crossref, Indexed at
- Panchendrabose K, Muram S, Mitha AP. Promoting endothelialization of flow-diverting stents: a review. J Neurointer Surg. 2021;13(1):86-90.
Google Scholar, Crossref, Indexed at
- Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38(8):2346-2352.
Google Scholar, Crossref, Indexed at
- Pai AM, Kameda-Smith M, van Adel B. A review of recent advances in endovascular therapy for intracranial aneurysms. Crit Rev Biomed Eng. 2018;46(4):369-397.
Google Scholar, Crossref, Indexed at
- Ignacio A, Sarabia R, Pintado R, et al. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurg. 2013;73(2):193-200.
Google Scholar
- Chen Y, Howe C, Lee Y, et al. Microstructured thin film nitinol for a neurovascular flow-diverter. Sci Rep. 2016;6:23698.
Google Scholar, Crossref, Indexed at
- Giacomini L, Piske RL, Baccin CE, et al. Neurovascular reconstruction with flow diverter stents for the treatment of 87 intracranial aneurysm: Clinical results. Interv Neuroradiol. 2015;21(3):292-299.
Google Scholar, Crossref, Indexed at
- Pierot L. Flow diverter stents in the treatment of intracranial aneurysms: Where are we? J Neuroradiol. 201;38(1):40-46.
Google Scholar, Crossref, Indexed at
- Indolfi C, De Rosa S, Colombo A. Bioresorbable vascular scaffolds — basic concepts and clinical outcome. Nat Rev Cardiol. 2016;13(12):719-729.
Google Scholar, Crossref, Indexed at
- Uurto I, Mikkonen J, Parkkinen J, et al. Drug-eluting biodegradable poly-D/L-lactic acid vascular stents: an experimental pilot study. J Endovasc Ther. 2005;12:371-379.
Google Scholar, Crossref, Indexed at
- Arat A, Daglioglu E, Akmangit I, et al. Bioresorbable vascular scaffolds in interventional neuroradiology. Clin Neuroradiol. 2018;28(4):585-592.
Google Scholar, Crossref, Indexed at
- Joost DV, Jeroen B, Anouk VN, et al. New generation of flow diverter (Surpass) for unruptured intracranial aneurysms. Stroke. 2013;44:1567-1577.
Google Scholar, Crossref






















