Research Article - (2022) Volume 0, Issue 0
Development of Neurotoxicity Syndrome Associated With the Use of Cefepime
Jhon Fredy Bello Cordero*
1Emergency Physician, Fundación Universitaria de Ciencias de la Salud, Bogotá, Colombia
Specialist in Critical Medicine and Intensive Care, Fundacion Universitaria de Ciencias de la Salud, Bogota, Colombia
General Physician, Universidad del Magdalena, Santa Marta Colombia, Colombia
General Physician, Universidad del Sinu, Monteria, Colombia
General Physician, Fundacion Universitaria San Martin, Colombia
General Physician, Universidad del Sinu, Cartagena, Colombia
General Physician, Universidad de Sucre, Sincelejo, Colombia
General Physician, Fundacion Universitaria San Martin, Colombia
*Correspondence:
Jhon Fredy Bello Cordero, Emergency Physician, Fundación Universitaria de Ciencias de la Salud, Bogotá,
Colombia,
Email:
Received: 11-Mar-2022, Manuscript No. Iphsj-22-12656;
Editor assigned: 13-Mar-2022, Pre QC No. Preqc No. PQ-12656;
Reviewed: 27-Mar-2022, QC No. QC No. Q-12656;
Revised: 01-Apr-2022, Manuscript No. Iphsj-22-12656(R);
Published:
09-Apr-2022, DOI: 10.36648/1791-809X.16.S6.921
Abstract
Cefepime is a broad-spectrum antibiotic; belonging to the group of fourth-generation cephalosporin’s commonly used in the hospital setting for the management of multiple infections. Due to mechanisms not well elucidated to date, it is capable of crossing the blood-brain barrier and causing adverse neurological effects when its plasma concentration rises, mainly in those patients in whom its dosage adjusted to creatinine clearance or creatinine clearance is not taken into account. Kidney functions if it is compromised. Also, cases have been reported in which patients with adequate renal function may present this heterogeneous presentation syndrome. The comprehensive management of the patient with this clinical presentation starts from prevention, correct dosage, and timely follow-up and early recognition of the Etiology of the neurological condition to obtain the best possible results for the same patient.
Keywords
Cefepime, Seizures, Neurotoxicity symptoms, Encephalopathy
Introduction
There is a wide variety of medications in the hospital setting for the management of different infections caused by bacteria. Today, one of the most common, due to its broad spectrum and decreased resistance profile, is Cefepime, a fourth-generation cephalosporin that covers both gram-positive and gram-negative bacteria. Like any other drug, it has adverse effects that should be known to the clinician when using it. Among them, CefepimeInduced Neurotoxicity, a rare but serious complication, represents a heterogeneous syndrome of neurological manifestations that can occur in patients receiving the antibiotic. One of the great challenges in clinical practice has been adjusting the dose of the medication according to individual renal compromise, since this is one of the risk factors most implicated in the development of adverse neurological effects. Additionally, adequate knowledge of the extension studies and their interpretation will allow better management of patients and avoid added side effects due to the association with other medications. In this short review, the clinical spectrum of Cefepime-Induced Neurotoxicity will be discussed; the importance of dose adjustment according to renal clearance, and management will be briefly discussed according to the clinical data found [1-3]
Methodology
A systematic bibliographic search of the updated medical literature on the development of neurotoxicity during the use of Cefepime was carried out, using databases such as: PubMed Science direct and Google Scholar. Descriptors such as cefepime, cephalosporin’s, seizures, neurotoxicity, and encephalopathy were used. Both review and original articles were used, taking into account that their year of publication was less than 10 years.
Result
The parenteral broad-spectrum antibiotic Cefepime, approved for use since the 1990s, is part of the group of fourth-generation cephalosporin’s with anti-pseudomonas activity and stability against certain extended-spectrum B-lactamases, commonly used for the management of multiple infections involving soft tissues, abdomen, skin, and urinary tract, due to either gram-positive or gram-negative bacteria in the hospital setting, including intensive care units [4-7]. Its primary excretion (85%) leads to the kidney and therefore patients with acute kidney injury, end-stage kidney disease and kidney transplants are at greater risk of developing complications associated with inappropriate use of the drug due to a decrease in the glomerular filtration rate and an increase in the serum levels of the drug that accumulates in the cerebrospinal fluid since it is capable of overcoming the blood-brain barrier due to an increase in its permeability, a decrease in serum binding to proteins and accumulation of organic acids that generate competitive inhibition to the GABA-a neurotransmitter. Despite the above, recent studies suggest that a significant proportion of patients with adequate renal function may also develop adverse neurological effects associated with Cefepime [8-12].
Cefepime-Induced Neurotoxicity (CIN) is a rare but lifethreatening complication that has been on the rise in recent years due to increased use of the antibiotic, increased resistance, and increased recognition of associated symptoms [13-15].
Although not fully understood, the pathophysiology of CIN is related to increased levels of Cefepime that cross the bloodbrain barrier, which leads to the appearance of CIN due to the high influx of the drug from the blood to the brain. Different mechanisms have been postulated, including that Cefepime has competitive antagonist activity for GABA-a, thus blocking/ inhibiting its receptor. Additionally, by decreasing the inhibitory activity, the presence of excitatory neurotransmitters is increased or the release of said neurotransmitter is inhibited. And, due to the above, an over excitation of electrical activity is generated that leads to the appearance of toxicity of the Central Nervous System [16- 20 ].
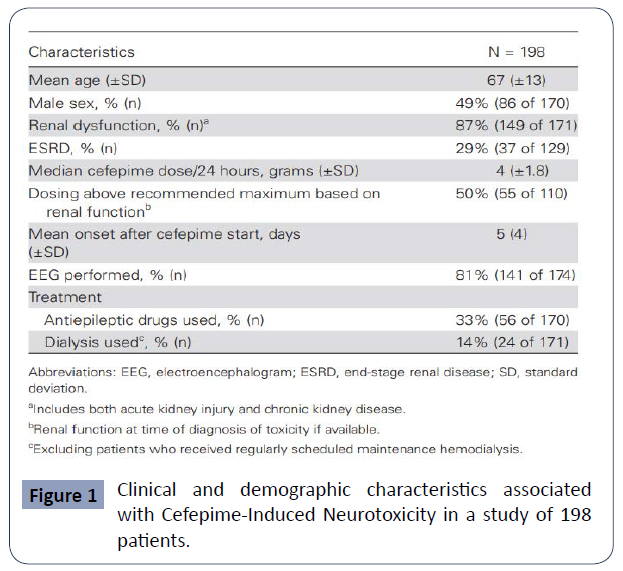
All of the above will lead to a broad neurological clinical presentation in patients, including altered mental status, confusion, encephalopathy, no convulsive status epilepticus (NCSE), seizures, asterixis, and coma [20-22]. Its incidence represents about 1-3% and a systematic review reported a single center estimate of one in 480 cases and concludes that CIN should be considered in patients with advanced age, renal dysfunction (particularly end-stage renal disease). and newonset encephalopathy, especially with concurrent myoclonus [1]. (Figure 1).It is important to keep in mind the variability of presentation of CIN. In the literature, it is considered a heterogeneous neurological syndrome that must be known to make an accurate and assertive diagnosis [1]. Encephalopathy is described as brain dysfunction of different aetiologies with changes in alertness and higher functions; it is considered to have a metabolic Etiology when a systemic trigger generates its presentation, whether due to toxic metabolites, neurotransmitters, post capillary vasogenic edema, or other mechanisms [23, 24].
Figure 1 Clinical and demographic characteristics associated with Cefepime-Induced Neurotoxicity in a study of 198 patients.
On the other hand, the presence of seizures and acute confusion have been reported in 1/10,000 cases and 1/1,000 cases, respectively (McEwen 2018). Like the rest of the neurological symptoms, although it is not totally clear, it is presumed that the seizures that occur due to cephalosporin’s occur due to the antagonistic effects of drugs on the GABA-receptors, which decreases the normal inhibitory response mediated by GABA and decreases the seizure threshold. This seizure activity can be effectively controlled with the use of benzodiazepines [25].
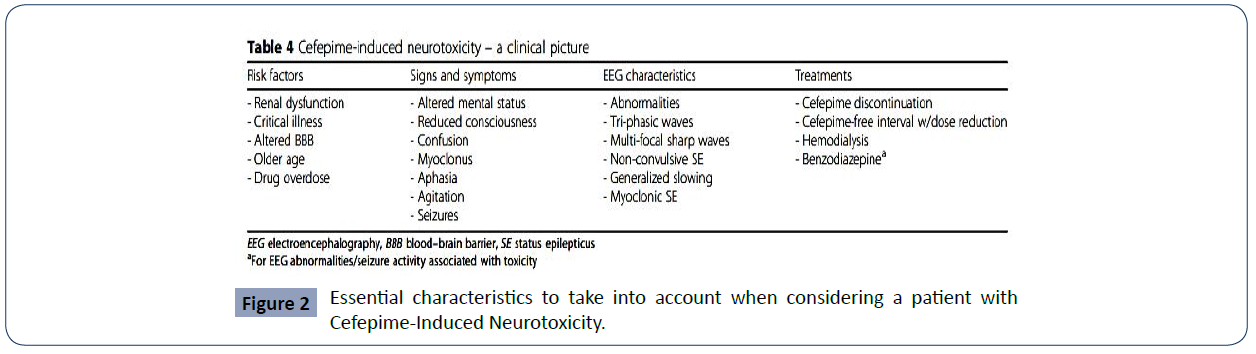
One of the entities with the greatest difficulty in its diagnosis is NCSE. It is defined as the presence of 30 minutes of continuous epileptiform activity associated with changes in mental status, but without motor manifestations. For its diagnosis, the use of the electroencephalogram (EEG) becomes relevant, which, on many occasions, is not readily available or is not used due to ignorance of the entity [1]. The early identification of the symptoms and their causal relationship with the use of the antibiotic and the definitive cessation of the administration of the medication are essential for the management of CIN. As adjuvant therapy, antiepileptic drugs (such as phenytoin, levetiracetam, benzodiazepines, and valproic acid) and the use of renal replacement therapy to reduce the plasma concentration of cefepime have been used in studies and considered useful [26-35]. Additionally, the most important preventive method for this heterogeneous neurological syndrome is described in the literature as strict attention to dosage adjusted to the patient's renal compromise, which would help health professionals in preventing morbidity and mortality associated with the use of the antibiotic. (Figure 2)
Figure 2 Essential characteristics to take into account when considering a patient with Cefepime-Induced Neurotoxicity.
Discussion
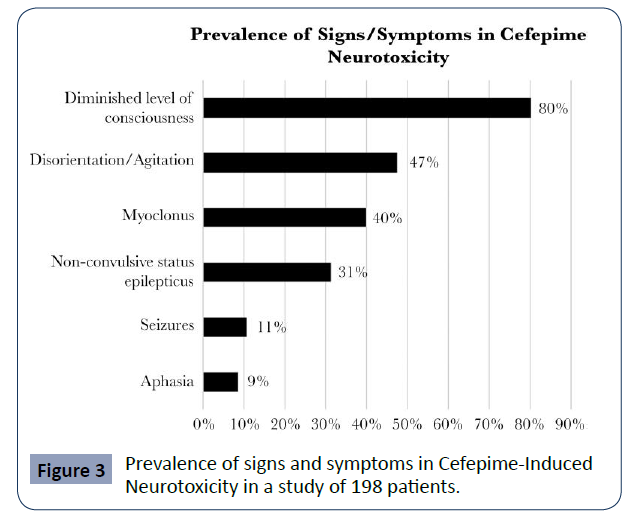
Appa, et al. conducted a systemic review of CIN in which they included 198 cases from the literature, including five that were part of their study centre. In it, they report that the mean age of the cases found for CIN is 67 years and most of them correspond to patients with renal dysfunction. It shows that most cases present with delirium or encephalopathy, 80% with decreased level of consciousness and 47% with disorientation and agitation. However, the presence of myoclonus can be seen in up to 40% of cases. The authors found that NCSE occurs in up to a third of cases, while only 11% present with seizure activity and 9% with aphasia. (Appa 2017) (Figure 3).
Figure 3 Prevalence of signs and symptoms in Cefepime-Induced Neurotoxicity in a study of 198 patients.
According to their pharmacological study, more than 85% of Cefepime is renally excreted through glomerular filtration and if creatinine clearance is compromised (<30ml/min), dose reduction for patient management is essential. To avoid both neurological and nephrological complications (McEwen 2018). The delay in its diagnosis can be associated with multiple comorbidities or the presence of several neurotoxic symptoms, mainly in patients managed in the Intensive Care Unit, where Fugate, et al. showed that many of the symptoms are common in critically ill patients and their relationship with drug use is taken into account as a rule-out diagnosis.
It has been shown in the literature that neurological complications generally occur within a median period of seven days (range 1-24 days) after treatment with Cefepime and that a median period of 3 ± 5 days elapses between treatments. onset of symptoms and possible diagnosis of CIN
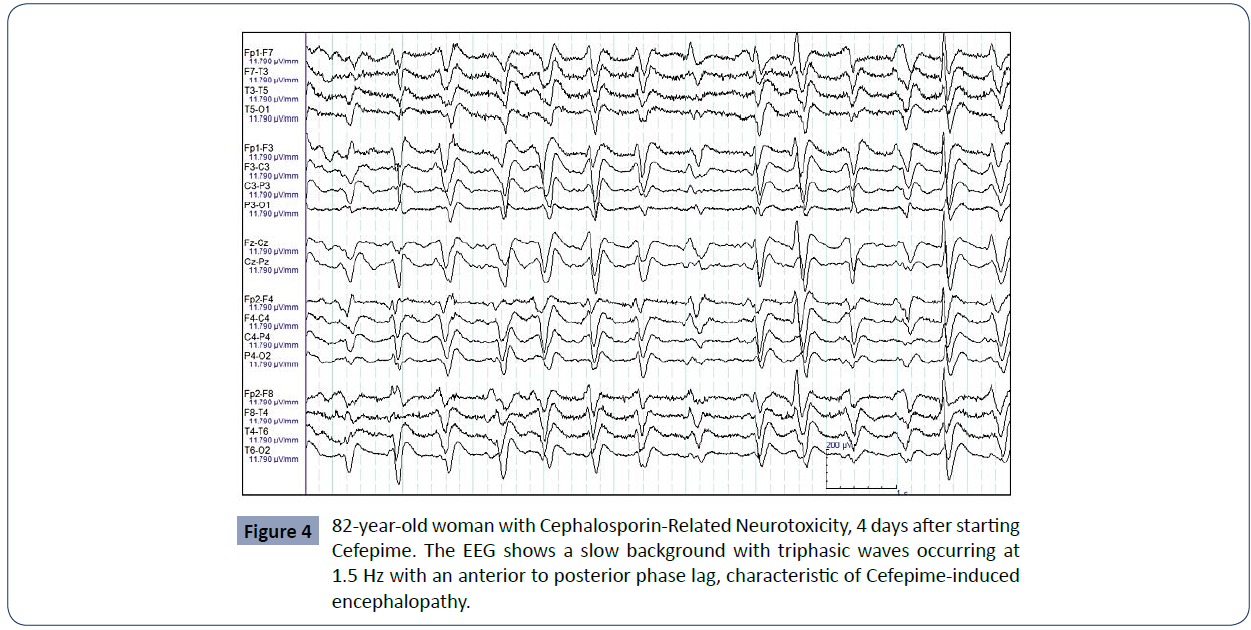
Concerned that the existing dichotomy between the term Cefepime-induced encephalopathy and NCSE caused by the drug affects the comprehensive management of patients, Tchapyjnikov, et. They studied four cases from their hospital center that developed alterations in their mental capacity associated with the use of Cefepime with triphasic discharges in the EEG, classic of a non-ictal pattern, and other cases found in the literature in which none responded adequately to the use of anticonvulsants. Retrospectively, they were able to realize that these patients, despite the use of benzodiazepines and even, in some of them with partial improvement of symptoms, only had normalization of mental status when Cefepime was discontinued, and they exhort to describe these specific cases as an encephalopathy induced by Cefepime and not by an ictal phenomenon, which allows a less aggressive treatment with the suspension of the antibiotic and without the use of anticonvulsants. Proper diagnosis of the adverse neurological effect caused by the drug would then allow avoiding additional drug side effects that compromise the patient, without the benefits of an adequate clinical response (Figure 4)
Figure 4 82-year-old woman with Cephalosporin-Related Neurotoxicity, 4 days after starting Cefepime. The EEG shows a slow background with triphasic waves occurring at 1.5 Hz with an anterior to posterior phase lag, characteristic of Cefepime-induced encephalopathy.
Adjusting the dosage in patients with kidney disease, whether acute or chronic, becomes essential when prescribing Cefepime for in-hospital management. One study demonstrated that accurate drug exposure (ie, pharmacokinetic/ toxicodynamics relationship) is key to preventing adverse neurological outcomes associated with drug use while maximizing drug efficacy. The authors questioned the minimum concentration of >22mg/L (T>22) found in the study by as a neurotoxicity threshold, who defined it as there must be at least a 50% probability of CIN if said threshold is exceeded. In this way, through a probabilistic analysis, they demonstrated that T>22 has a low accuracy for CIN in patients with "common" dosages with readjustment according to their creatinine clearance, considered "low risk" for neurotoxicity, and in him , 5-48% of patients will experience CIN. Therefore, such is an insufficient parameter to predict toxicity and further studies are required to elucidate the exact exposure profile to Cefepime. Recently, a study observed that the minimum plasma concentration of Cefepime ≥36 mg/L differentiates those who develop neurotoxic side effects from those who do not.
Even despite the renal adjustment of the drug for its use, cases have been published in which neurotoxicity syndrome occurs, mainly in elderly patients Park, et. al, published a case in which a 74-year-old woman with a history of arterial hypertension and creatinine clearance calculated by MDRD of 87 mL/min/1.73m2 (normal renal function), who, on the seventh day after starting management in-hospital treatment with Cefepime due to osteomyelitis in the recurrent right mandible began with general weakness and drowsiness and on the ninth day with a stuporous mental state, tonic spasms in the arms and irregular spasmodic movements of the lower limbs. Subjected to extensive imaging studies without conclusive findings, the clinicians performed an electroencephalography finding spikes and continuous rhythmic wave’s characteristic of Non-Convulsive Status Epilepticus. The antibiotic was withdrawn, antiepileptic drugs were started and after 72h of management, the patient gradually recovered her normal neurological activity. Strict monitoring of creatinine clearance should also not be forgotten since, additionally, Nephrotoxicity due to Cepefime may also occur that worsens or triggers the CIN picture.
Likewise, there are also cases in which, despite dose correction adjusted for renal creatinine clearance, signs and symptoms of CIN may occur, such as the one presented in which a 64-year-old woman years old, with multiple comorbidities and a history of kidney transplantation 18 years earlier, he was admitted to the hospital with symptoms associated with acute kidney injury and, despite fluid and electrolyte replacement therapy, his kidney function slowly deteriorated, which was initially considered acute rejection. Chronic transplant. During her management, she developed Pseudomonas aeruginosa infection for which adjusted management with Cefepime was started. On the sixth day of treatment, she started with symptoms consistent with neurotoxicity and the EEG study reported subclinical status epilepticus. The patient improved markedly after discontinuation of the antibiotic.
For all the above, it is necessary for the clinician to have a high index of suspicion and awareness of the disease for a timely diagnosis, adequate and assertive treatment that can help in the recovery of patients.
Conclusion
Cefepime-Induced Neurotoxicity is a rare but life-threatening complication that should be known by all clinicians and should be considered in all patients with impaired neurological function who are receiving the drug, regardless of their renal function status. To reduce its incidence, alternative antibiotics should be used, make an appropriate dose adjustment according to the patient's renal function and take the entity into account for timely and assertive diagnosis and treatment. Further studies are needed to understand the risk factors, pathophysiology, and true pharmacokinetics/toxicodynamics to be considered when using it in patients.
REFERENCES
- Appa A A, Jain R, Rakita R M, Hakimian S, Pottinger P S (2017) Characterizing Cefepime Neurotoxicity: A Systematic Review. Open Forum Infect Dis 4:ofx170.
Indexed at, Google scholar, Crossref
- Park HM, Noh Y, Yang JW, Shin DH, Lee YB (2016) Cefepime-Induced Non-Convulsive Status Epilepticus in a Patient with Normal Renal Function. J Epilepsy Res 6:97-99.
Indexed at, Google scholar, Crossref
- McEwen Tamayo O, Blaguera P, Montes M, Hernández O (2018) Neurotoxicidad asociada a cefepime, reporte de un caso y revision de la literature. Acta Colomb Cuid Intensivo
Indexed at, Google scholar, Crossref
- Payne LE, Gagnon DJ, Riker RR, Seder DB, Glisic EK et al. (2017) Cefepime-induced neurotoxicity: a systematic review. Crit Care 21:276.
Indexed at, Google scholar, Crossref
- Lindsay H, Gruner S, Brackett J (2017) Cefepime-Induced Neurotoxicity despite Dose Adjustment for Renal Disease: A Brief Report and Review of the Literature. J Pediatric Infect Dis Soc 6:199-201.
Indexed at, Google scholar, Crossref
- Tchapyjnikov D, Luedke MW (2019) Cefepime-Induced Encephalopathy and No convulsive Status Epilepticus: Dispelling an Artificial Dichotomy. Neurohospitalist 9:100-104.
Indexed at, Google scholar, Crossref
- Triplett JD, Lawn ND, Chan J, Dunne JW (2019) Cephalosporin-related neurotoxicity: Metabolic encephalopathy or non-convulsive status epilepticus? J Clin Neurosci 67:163-166.
Indexed at, Google scholar, Crossref
- Saini T, Gaines MN, Sohal A, Li L (2021) Cefepime-Induced Neurotoxicity. Cureus 13:e17831.
Indexed at, Google scholar, Crossref
- Oyenuga M, Oyenuga A, Rauf A, Balogun O, Singh N Â (2021) New Onset Non-Convulsive Status Epilepticus Despite Cefepime Renal Dose Adjustment. Cureus 13:e12689.
Indexed at, Google scholar, Crossref
- Li HT, Lee CH, Wu T, Cheng MY, Tseng WJ (2019) Clinical Electroencephalographic Features and Prognostic Factors of Cefepime-Induced Neurotoxicity: A Retrospective Study. Neurocrit Care 31:329-337.
Indexed at, Google scholar, Crossref
- Lichak BP, Lawal O, Polimera HV, Garg A, Kaur G (2021) A Case of Cefepime-Induced Neurotoxicity: Renal Function Missing in Action. Cureus 13:e13368.
Indexed at, Google scholar, Crossref
- Rhodes NJ, Kuti JL, Nicolau DP, Neely MN, Nicasio AM et al. (2016) an exploratory analysis of the ability of a cefepime trough concentration greater than 22 mg/L to predict neurotoxicity. J Infect Chemother 22:78-83.
Indexed at, Google scholar, Crossref
- Boschung-Pasquier L, Â Atkinson A, Kastner LK, Banholzer S, Haschke M (2020) Cefepime neurotoxicity: thresholds and risk factors. A retrospective cohort study. Clin Microbiol Infect 26:333-339.
Indexed at, Google scholar, Crossref
- Lau C, Marriott D, Gould M, Andresen D, Reuter SE et al. (2020) A retrospective study to determine the cefepime-induced neurotoxicity threshold in hospitalized patients.  J Antimicrob Chemother  75:718-725.
Indexed at, Google scholar, Crossref
- Cindy L A U, Marriott D, Schultz H B, Gould M, Andresen D (2021) Assessment of cefepime toxico-dynamics: comprehensive examination of pharmacokinetic/pharmacodynamics targets for cefepime-induced neurotoxicity and evaluation of current dosing guidelines. Â Int J Antimicrob Agents, 106443. 2021.
Indexed at, Google scholar, Cross Ref
- Lee S J (2019) Cefepime-induced neurotoxicity. J Neurocrit Care 12:74-84.
Google scholar, Crossref
- Roger C, Louart B (2021) Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms, 9:1505.
Indexed at, Google scholar, Cross Ref
- Ortega AJ, Ghafouri SR, Vu L, Edwards B, Nickel N (2021) Cefepime-Induced Encephalopathy in a High-Risk Patient With Renal Insufficiency and Cirrhosis. Cureus 13:e18767.
Indexed at, Google scholar, Crossref
- Tamune H, Hamamoto Y, Aso N, Yamamoto N (2019) Cefepime-induced encephalopathy: Neural mass modeling of triphasic wave-like generalized periodic discharges with a high negative component (Tri-HNC). Psychiatry Clin Neurosci 73:34-42.
Indexed at, Google scholar, Crossref
- Somoza-Cano FJ, Al Armashi AR, Weiland A, Chakhachiro D, Ravakhah K (2021) Cefepime-Induced Delirium. Cureus 13:e15505.
Indexed at, Google scholar, Crossref
- Keerty D, Shareef NA, Ramsakal A, Haynes E, Syed M (2021) Cefepime-Induced Encephalopathy. Cureus 13:e13125.
Indexed at, Google scholar, Crossref
- Jeon JY, Cho YW, Moon HJ (2020) Cefepime-Induced Encephalopathy in a Tertiary Medical Center in Korea. J Clin Neurol 16:408-415.
Indexed at, Google scholar, Cross Ref
- Behal M L, Thomas J K, Thompson Bastin M L, Mefford B M (2021) Cefepime Induced Neurotoxicity Following A Regimen Dose-Adjusted for Renal Function: Case Report and Review of the Literature. Hospital Pharmacy 00185787211046856.
Google scholar, Crossref
- DAS S K, DAS S K, PANDA A, PARHI L (2021) Cefepime Induced Neurotoxicity: Case Series. J clin diagn 15.
- Sandhu G, Magorien J (2020) Cefepime Induced Neurotoxicity. Proceedings of UCLA Health 24
Indexed at, Google scholar
- Cunningham J M, Sachs K V, Allyn R (2020) Cefepime-Induced Neurotoxicity Presenting with Nonconvulsive Status Epilepticus Admitted as a Stroke Alert. Am J Med Case Rep 21:e921643-1.
Indexed at, Google scholar, Crossref
- Oda K, Miyakawa T, Katanoda T, Hashiguchi Y, Iwamura K (2020) case of recovery from aphasia following dose reduction of cefepime by bayesian prediction-based therapeutic drug monitoring. J Infect Chemother 26:498-501.
Indexed at, Google scholar, Crossref
- Venugopalan V, Nys C, Hurst N, Chen Y, Bruzzone M (2020) Use of Therapeutic Drug Monitoring to Characterize Cefepime-Induced Neurotoxicity bioRxiv.
Indexed at, Google scholar, Crossref
- Sekhar S M (2020) Cefepime-Induced Neurotoxicity A Retrospective Cohort Study in a Tertiary Healthcare Facility.
Google scholar
- Khorasani-Zadeh A, Greca I, Gada K (2020) Cefepime-Induced Seizures: The Overlooked Outpatient Adverse Reaction. Cureus 12.
Indexed at, Google scholar, Cross Ref
- Vercheval C, Sadzot B, Maes N, Denooz R, Damas Pet al. (2021) Continuous infusion of cefepime and neurotoxicity a retrospective cohort study CMI 27:731-735.
Indexed at, Google scholar, Crossref
- Al-Shaer M H, Peloquin C A (2021) Using precision dosing to minimize cefepime-induced neurotoxicity: the challenge of targets: official journal of the Japan Society of Chemotherapy. J Infect Chemother 27:929-930.
Indexed at, Google scholar, Crossref
- Fernandez-Fernández F J, Ameneiros-Lago E (2020) Cefepime-Induced Encephalopathy A Possible Additional Mechanism of Neurotoxicity. J Neurocrit Care 32:641-641.
Indexed at, Google scholar, Crossref
- Leonard S D (2020) Cefepime-induced Encephalopathy and Neurotoxicity. Proceedings of UCLA Health 24.
Google scholar
- Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, et al. (2010) High cefepime plasma concentrations and neurological toxicity in febrile neutropenia patients with mild impairment of renal function. Antimicrob Agents Chemother 54:4360-4367.
Indexed at, Google scholar, Crossref
Citation: Citation: Cordero JFB, Lopez JAM, Lora JAF, Martinez JMM, Mercado LPN, et al. (2022) Development of Neurotoxicity Syndrome Associated With the Use of Cefepime. Health Sci J. Vol. 16 No. S6: 921.