Review Article - (2023) Volume 14, Issue 5
Development of new anticancer virus-based treatments: A comprehensive review
Namra Ahmad1,
Muhammad Hamza Ashraf1*,
Nazim Hussain1*,
Muhammad Ikram Ramzan2,
Qandeel Bajwa3,
Sidra Manawar4,
Muhammad Rizwan Ali2,
Muhammad Asif Muneer1,
Muhammad Zafar Saleem1 and
Syed Muhammad Asad Ali1
1Center for Applied Molecular Biology (CAMB), University of the Punjab, Lahore, Pakistan
2National Centre of excellence in Molecular Biology (CEMB), University of the Punjab, Lahore, Pakistan
3Department of Biotechnology, University of Central Punjab, Lahore, Pakistan
4Department of Pathology, Gujranwala Medical College, Gujranwala, Pakistan
*Correspondence:
Muhammad Hamza Ashraf, Center for Applied Molecular Biology (CAMB), University of the Punjab, Lahore,
Pakistan,
Email:
Nazim Hussain, Center for Applied Molecular Biology (CAMB), University of the Punjab, Lahore,
Pakistan,
Email:
Received: 11-Aug-2023, Manuscript No. ipacm-23-14022;
Editor assigned: 14-Aug-2023, Pre QC No. ipacm-23-14022 (PQ);
Reviewed: 28-Aug-2023, QC No. ipacm-23-14022;
, Manuscript No. ipacm-23-14022 (R);
Published:
04-Dec-2023
Abstract
Oncolytic virotherapy has turned into a noteworthy cancer therapeutic approach, preferentially targeting and killing cancerous cells without affecting healthy cells. Oncolytic viruses' propensity to proliferate specifically in tumor cells results in rapid cell disruption and the development of an aggressive antitumor immune reaction. The advances in molecular biology and biotechnology approaches for genetic manipulation of recombinant viruses for clinical applications have made real progress. Modifications in the genetic sequence of oncolytic viruses can enhance cell-targeting efficacy. This evolving research has resulted in improvements in the efficacy and specificity of OVs as an alternative therapeutic approach for malignant tumours, albeit with significant methodological flaws. Nevertheless, it has become obvious that oncolytic viruses alone may not be able to offer a comprehensive response for cancer treatment, necessitating the use of combinatorial techniques. Emerging multimodality treatment strategies could include oncolytic virotherapy along with other anti-tumor therapies involving chemotherapy, CAR-T cell therapies, radiotherapy, Immune Checkpoint Inhibitors (ICIs) and bi-specific T cell engagers. Various regulatory systems, like autophagy, assist in the anti-tumor capabilities of oncolytic viruses by boosting their anti-tumor activities through activation of oncolysis, immunogenicity and autophagic cell death. Such combinatorial therapies with oncolytic viruses could be useful for further enhancing treatment consequences as multiple component strategies can report the errors of every component. The intrinsic constraints of oncolytic viruses and other drugs against certain types of cancer can be mitigated by a sensible genetic design and a combination strategy.
Keywords
Oncolytic viruses; Cancer treatment; Autophagy; OVs;
Antitumor response; Combinatorial therapy.
Introduction
According to World Health Organization (WHO)
estimates, cancer is currently second biggest cause of
death globally, accounting for approximately one-sixth of
all fatalities. In 2021, the United States is expected to see
1,898,160 new cases of cancer and 608,570 cancer deaths.
Due to declines in smoking and advancements in early
identification and treatment, the death rate from cancer
has declined steadily from its high in 1991 to 2018, a
total reduction of 31% [1]. Cancer treatment is difficult,
and experts are exploring novel ways to combat the
disease. Conventional targeted therapies such as surgeries,
hormone therapy, chemotherapy and radiotherapy have
poor outcomes due to immunosuppression induced by
tumor and multidrug resistance, which increased the risk
of cancer spread and recurrence [2-4].
Viruses and virus inspired platforms have recently gained
attention as a potential way to overcome the drawbacks
of conventional cancer treatment. The deliberate use of
viruses for cancer therapy has a lengthy history, extending
back to the 1890’s at the very least. Following the success
of Streptococcus pyogenes bacteria in patients with
severe bacterial infections, surgeon William Coley gave
Streptococcus pyogenes bacteria to a patient with nonoperative
bone carcinoma, resulting in tumor shrinkage
[5]. Many incidences of tumor regression were recorded
during the next several decades because of both viral and
microbial infection. In 1904, for example, a cervical cancer
patient was given a live attenuated rabies vaccine to treat a
dog bite wound. The tumor mysteriously vanished, and
the patient remained free from cancer for the following
eight years, much to the surprise of the doctors. Soon after,
the administration of rabies vaccine to eight more cervical
cancer patients, some of whom observed their tumors decline
[6]. However, considerations regarding viral pathogenicity
and toxicity made SSS this strategy unfeasible. Oncolytic
viruses are a new breed of viruses developed by recent
developments in genetic engineering methods that assure
their effectiveness and safety. Oncolytic viruses, which are
replication competent viruses that specifically proliferate in
tumor cells and kill them without harming healthy cells,
are intriguing cancer candidate therapy. By lysing tumor
cells and releasing DAMPs (Damage Associated Molecular
Patterns), TAA (Tumor Associated Antigens) and PAMPs (Pathogen Associated Molecular Patterns), oncolytic viruses
can enhance anti tumor immune responses, resulting in
the induction of Antigen Presenting Cells (APCs) and
activation of adaptive immune responses.
Literature Review
Oncolytic viruses
Oncolytic virotherapy is based on the idea of treating cancer with oncolytic viruses that particularly replicate and induce cancer cells apoptosis while preventing healthy tissues [7]. OVs are an excellent platform for treating cancer. By modifying the viral genome to improve the selective replication of virus and lytic capacity, enhance viral tropism to neoplastic cells and boost the antitumor immunity of the host [8]. These characteristics are the basis for using OVs to treat cancer. OVs can modify virus to stimulate antitumor immunity, improve sensitivity of tumor towards radiation therapies or traditional treatments, and ensure patient safety [9].
Classification of oncolytic viruses
Reovirus: Reovirus is a double stranded and non-enveloped RNA virus which replicates selectively in transformed cells not in naive cells. It belongs to the Reoviridae family [10]. Upregulation of growth factor signals and RAS signaling pathway overexpression in target cells are both involved in reovirus oncolysis [11,12]. The activation of PKR is blocked by EGFR (activated epidermal growth factor receptor) and hyperactive RAS, permitting viral protein production and effective lytic infection that leads to cell lysis. Reolysin is an attenuated reovirus Type 3 Dearing (T3D) strain that has been extensively studied as an anticancer drug and is the only wild type strain now in clinical trials [13]. The first phase I clinical study for reovirus used intralesional monotherapy to treat 19 patients with advanced solid tumours. Treating with progressive doses up to 1010 PFU was shown to be well tolerated and safe, with no dose limiting toxicities [14]. Another phase I multicenter trial was conducted in 15 patients with recurrent malignant gliomas who received reovirus by intratumoral infusion for 72 hours at five distinct dose ranges varying from 1 £ 108 to 1 £ 1010 Tissue Culture Contagious Dose 50 percent (TCID50). The study found that reovirus administered intra tumorally was well accepted and tolerated as a monotherapy [15]. A phase II trial was also carried out in those patients with metastatic melanoma who were given reovirus intravenously at a dose of 3 $ 1010 TCID50 for every hour on days 15 and every four weeks. Although substantial tumor necrosis of 75 to 90 percent was documented in one patient having metastatic tumours surgically excised, no objective responses were detected [16].
Measles virus: Measles Virus (MV) is a negative stranded RNA (16 kb) and enveloped paramyxovirus that contains six genes encoding eight viral proteins. At both the entrance and post entry phases, MV strains conferred tumor selectivity. The virus enters the cell by interacting with the receptors of the host cell like Nectin-4, CD46 and SLAM (a Signaling Lymphocyte Activating Molecule) through its hemagglutinin H protein [17,18]. The wild type measles virus was more effective at entering cells via SLAM, but the Edmonston strain was more effective at entering cells via CD46 receptors [19]. CD46 is abundantly expressed in numerous cancer cells, allowing MV to enter and propagate more easily in tumor cells. In cancer cells, the type I IFN pathway, which makes them more favourable for viral replication, is one example of aberrations in the antiviral immune response at the post entry phase [20,21]. The C and V proteins encoded by MV prevent the production of IFN-a and b, allowing the replication of viruses in the host cell. Insertions of integrin binding peptides or receptors of single chain T-cells, envelope fusion protein modification, as well as tumor specific ligands, have all been reported to increase cancer specific tropism [22]. A recombinant Edmonston measles virus strain MV-NIS expresses the NIS (Sodium Iodide Symporter), enabling infected cell imaging and overseeing treatment development, as well as promoting I-131 uptake by infected cells [23]. MV-NIS is currently being tested in patients with ovarian and breast malignancies, mesothelioma and multiple myeloma in a number of clinical trials [24].
Enteroviruses: Picornaviruses are a single stranded, positive sense, large family of RNA viruses with icosahedral capsids of 30 nm. RNA viruses (non-enveloped) infect vertebrates, including birds and mammals and belong to this family. Over 300 different types of entero viruses have been identified, demonstrating heterogeneity and incredible diversity [25-28]. ECHO (Enteric Cytopathogenic Human Orphan) serotypes 1, 7 and 12, PVS-RIPO (modified poliovirus type 1 with human rhinovirus IRES region) and Coxsackievirus are among the most comprehensively studied in clinical studies [29-31]. Coxsackieviruses are classified as A or B, with serotype A21 being the most often utilised due to its cytotoxicity towards cancer cells [32]. Oncolytic activity of other serotypes, such as A13, A15 and A18, has also been investigated [33]. CVA21, a coxsackievirus, had significant oncolytic action against myeloma xenografts. Cavatak, a Coxsackievirus mutant, is being tested in conjunction with pembrolizumab and ipilimumab [34]. Poliovirus (Enterovirus genus), which causes paralytic poliomyelitis, is another member of this virus family that can be used in virotherapy. Within 6 hours of infection, the poliovirus produces roughly 10,000 mature virions per infected cell [35]. On the other hand, the use of wild type poliovirus has been linked to neurotoxicity. PVS-RIPO, a neuro attenuated poliovirus, was produced to reduce the risk. It was tested in grade IV malignant gliomas and found to have no neurotoxicity [36]. The oncolytic capability of entero viruses was initially discovered in the 1950’s [37]. The LEV-15L strain (Coxsackievirus B6) has been approved as a treatment for HPV-negative cervical cancer (RU 2496873). Coxsackievirus B5 and A7 have been employed to successfully kill glioblastoma stem cells and slow tumor growth [38,39]. A new delivery strategy using human NK-92 cells and dendritic cells (US2020/0352993) has been developed to decrease the immune response after oncolytic virus injection [40]. ECHO type 7 is another oncolytic enterovirus with prominent oncolytic activities [41]. After more directed development and selection, the oncolytic ECHO-7 virus was registered as Rigvir in Latvia in 2004. Glioblastoma, malignant glioma and melanoma are the most common cancers treated with enteroviruses [42].
Newcastle Disease Virus (NDV): Newcastle disease virus has an RNA genome that is negative stranded as well as non-segmented. It is not harmful to people and has been found to destroy tumor cells selectively. Infected cells are not sensitive to insertional mutagenesis because NDV replicates in the cytoplasm [43]. Due to a failure in the IFN pathway in cancer cells, NDV indicated selective replication in tumor cells [44]. Tumour cells contain a defective type I IFN pathway, which seems to be a crucial hallmark of tumorigenesis, making them more vulnerable to NDV infection. NDV promotes cancer cell death (apoptosis) and can transform an immunosuppressive tumor microenvironment into a proinflammatory one that promotes antitumor immunity [45]. Live attenuated NDV strains have been studied for treating malignancies in humans, including nonlytic lentogenic NDVHUJ as well as lytic mesogenic NDV strains like MTH68/H and PV701 [46]. After conventional treatments failed, MTH68/H was given to patients with the most aggressive and severe glioblastomas. Four of the treated individuals had survival rates of 5 to 9 years [47]. PV701 trials have highlighted concerns that repeated NDV administrations may lead the human immune system to produce virus-neutralising antibodies [48,49]. It may be possible to create a more efficient recombinant NDV vaccination utilising reverse genetics techniques. To improve the anticancer effects and immunogenic potential of NDV-based vaccines, genetic engineering of the virus with transgenes encoding tumoricidal compounds or cytokines is being studied [50,51].
Adenoviruses: Adenoviruses are non-enveloped with a linear double stranded DNA (dsDNA) genome of approximately 36 kb that comprises early genes (E1–E4) and late genes (L1–L5) [52]. Fibre, penton base and hexon are three key proteins found in adenoviruses (ads) that are accountable for cell infection. The arginine glycine aspartic acid (RGD) motifs on the penton base of the Ad allow the fibre protein to engage with cellular integrins and infect host cells after initially binding to the Coxsackie and Adenovirus Receptor (CAR) [53]. In 2005, China approved Oncorine (H101), an oncolytic adenovirus with four deletions in the E3 gene and one deletion in the E1B-55k gene, for the treatment of neck and head cancers [54]. Oncorine selectively replicates in p53-deficient tumor cells because E1B-55k is a potent p53 repressor [55]. Moreover, in Rp-deficient tumours, a 24 bp loss in the E1A gene's CR2 domain, which is responsible for binding to the Retino blastoma Protein (pRb) or its family members, restricts Ad replication [56,57]. For targeting tumor cells, several oncolytic ads have been produced with a deletion in the E1ACR2 region. For example, DNX-2401 (Delta-24-RGD) is armed with an RGD-4C integrin targeting sequence in addition to a deletion in the E1ACR2 region to target cancer cells [58]. ONCOS-102 (Ad5/3-D24-GM-CSF) is an Ad Delta-24 chimeric capsid equipped with granulocyte Macrophage Colony Stimulating Factor (GM-CSF) to boost immune stimulatory effects [59]. The Ad5 fibre knob domain has been substituted with an Ad3 fibre knob domain in the Ad5/3 chimeric capsid for improved cancer cell targeting due to CAR downregulation in advanced tumours [60].
Herpes simplex viruses: HSV-1 (HSY type 1) is an enveloped dsDNA virus with a 152 kb genome. About 84 genes are encoded by the DNA genome, which is made up of /Late (L), /Early genes (E) and /Immediate-Early (IE) genes [61]. The only FDA approved oncolytic virus, T-VEC, is an engineered HSV-1 that enters tumor cells via nectins and replicates by tolerating distorted antiviral and oncogenic pathways, including the IFN (type I interferon) and PKR (protein kinase R) pathways [62]. The RL1 gene encodes a neuro virulence factor (ICP34.5) protein that is required for effective infection in healthy cells [63]. T-VEC has been further modified by removing the US12 gene, which produces the viral ICP47 protein, to improve antigen presentation and T-cell priming. By binding to TAP and blocking antigen loading of MHC-1 molecules, this protein decreases the immunological death of HSV-1-infected cells [64]. HF10 (Canerpaturev) is a naturally modified HSV-1 with oncolytic capabilities [65]. High viral multiplication, high tumor selectivity, powerful antitumor effects against tumor cells, the beginning of a cytopathic impact and the intermediation of the bystander effect are all caused by these natural genetic modifications in HF10 [66].
Vaccinia virus: Vaccinia viruses, which are double stranded DNA viruses with an envelope and belong to the Poxviridae family, were used as vaccinations to eradicate smallpox [67]. Numerous virulence genes are included in the vaccinia virus. Most of them operate in one of three ways: Intracellularly, primarily to inhibit apoptotic pathways; externally, primarily to activate or suppress cell signalling pathways; or thirdly, primarily by secreting decoy receptors that sequester cytokines and chemokines from the extracellular environment, to suppress the immune response to the virus. Many of these virulence characteristics also target the hallmarks of cancer, so their deletion will attenuate the virus primarily in non-tumor cells and produce an oncolytic drug with improved selectivity and stronger therapeutic index [68]. As a result, their functions are frequently redundant in cancer cells. The GM-CSF-expressing vaccinia strain JX-594 is currently in phase III clinical testing after demonstrating promising results in phase I studies for hepatocellular tumours [69]. One benefit of using vaccinia as an oncolytic agent in this field is that numerous immunomodulatory transgenes may be expressed from the same vector [70]. Because its absence leads in a virus that is dependent on cellular thymidine kinase, an enzyme that is frequently overexpressed in cancer cells, the viral thymidine kinase gene has been engineered the most frequently into oncolytic vaccinia strains [71]. Replication of this virus can be further restricted to cancer cells by combining viral growth factor gene deletion [72,73]. Although this virus has a potent oncolytic effect and is primarily restricted to reproducing in malignant cells, the ongoing expression of the virus' immunosuppressive virulence genes may reduce its potential for immunotherapy. In order to specifically target cancer cells and express the therapeutic transgene Granulocyte Macrophage Colony Stimulating Factor (GM-CSF), which encourages antitumor immunity, Pexa-Vec is created from a strain of the vaccinia vaccine. The ability of the vaccinia virus to affect metastatic disease depends on the emergence of specific features that allow for effective systemic spread. In preclinical models and human patients, Pexa-Vec has been found to have a number of various Modes of Action (MOA), including tumour cell infection and lysis, antitumor immune response stimulation and tumour vascular disruption. Enzyme Linked Immuno Spot (ELISPOT) research revealed that Pexa Vec treated HCC patients had T cell responses to galactosidase peptides; this serves as proof of concept that T cell responses can be triggered to transgenes encoded by oncolytic vaccinia viruses [67].
Anti tumor mechanism of OVS
OVs reproduce selectively in tumor cells, damage them via direct cell lysis, and activate anticancer immunological responses in the host [74]. Some OVs replicate in the cytoplasm of the host cells. Other OVs, such as adenovirus and HSV (and most other DNA oncolytic viruses), are replicated in the nucleus. OVs infect tumor cells and reproduce before lysing the cell to release progeny virions that infect nearby cells and cause the death of cancer cells. A few factors influence tumor selectivity in oncolytic virotherapy, including the virus's entrance through pathways mediated by receptors. Cancer cells have been shown to have high levels of expression of several distinct receptors that the viruses utilise to preferentially bind and infect the cells. Decay-accelerating factor and ICAM-1 (Intercellular Adhesion Molecule 1), for example, are highly expressed in many cancer cells infected by the coxsackievirus CV-A21 [75]. The measles virus was found to enter cells through overexpressed CD46 surface receptors in ovarian, colorectal and breast cancer cells [17,19]. HSV enters cells by nectin or herpesvirus entry mediator, although other viruses, like vaccinia and NDV, enter cells via endocytosis since they lack specialised receptors for attachment (Fig. 1). Tumour cells are targeted by OVs while protecting normal cells using a variety of strategies. Exploiting cancer cells abnormal signaling pathways could lead to the breakdown of viral defence mechanisms, allowing viral multiplication. Defects in the Retino Blastoma (Rb), p53 and Interferon (IFN) pathways, as well as stimulation of the RAS/RAF/MEK/ERK pathways, can cause changes in cancer cells [76,77]. For example, one of the basic mechanisms for viral replication (tumor-specific) in mostly OVs is impaired antiviral defence mechanisms in cancers, like IFN. By recognising viral components and activating toll like receptors, viral infection in normal cells stimulates the generation of type-1 interferon and the activation of various downstream pathways, including the stimulation of PKR (protein kinase R).
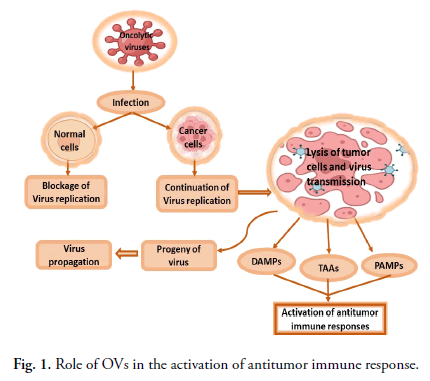
Fig 1. Role of OVs in the activation of antitumor immune response.
As a result, the phosphorylated PKR prevents the synthesis of viral proteins as well as replication in cells. Tumour cells, on the other hand, have aberrant PKR activity and the IFN pathway, which prevent virus clearance and increase vulnerability to virus reproduction [78]. Another typical hallmark of tumor cells is a fundamentally active RAS signaling pathway, which can prevent autophosphorylation of PKR and enable virus replication [79]. Natural selectivity for cancer cells is demonstrated by VV, HSY and reoviruses in the presence of an overactive RAS signaling pathway. Fig. 2 depicts the mechanisms of oncolysis of various OVs and tumor tropism. After viral replication, OVs cause cell death by releasing DAMP (Damage Associated Molecular Patterns), PAMP (Pathogen Associated Molecular Patterns) and cytokines (for example, IL-1, IFN-a, IL-6, TNF-a and IFN-g) that drive innate immune responses. Furthermore, after the lysis of tumours, TAAs (Tumor Associated Antigens) are released and antigen presenting cells present tumor antigen, resulting in an adaptive immune response and the activation of CD8+ and CD4+ T cells for tumor cell destruction [80]. The stimulation of innate and adaptive immunological responses by OVs, on the other hand, might be a double-edged weapon. OVs penetrate cells by interacting with certain surface receptors that are abundant in tumor cells. Viruses employ aberrant ways to multiply after getting entry into the cell. IFN (interferon) synthesis caused by infected cells activates the kinase signal transducer and activator of the AK/STAT signalling pathway in healthy cells, resulting in PKR activation. It further causes the phosphorylation of α subunit of eIF2 which will in turn stop the viral replication by inhibiting the viral protein formation. In tumorous cells, this interferon pathway is not regulated and as a result viral replication will continue. Different viruses employ this characteristic for their inherent oncotropism. Furthermore, excessive RAS signalling, which itself is typically seen in cancer cells, prevents the protein kinase R pathway, allowing OVs including vaccinia virus, Herpes Simplex Virus (HSV) and reovirus to replicate selectively. Certain viruses, like reovirus and adenovirus, choose to proliferate in p53-deficient tumor cells since normal cells with functional p53 die when they are infected. Due to abnormal signalling of the Retinoblastoma (Rb) route in cancerous cells, some OVs become able to multiply and destroy tumor cells that have uncontrolled E2F expression.
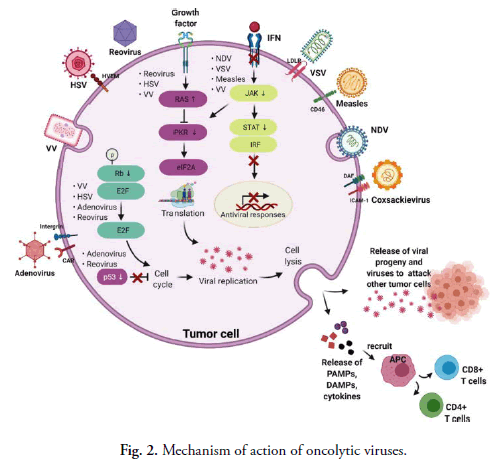
Fig 2. Mechanism of action of oncolytic viruses.
Approaches for the enhancement of therapeutic efficiency of OVs
To enhance the therapeutic efficiency of OVs, various effective methods have been used, primarily at two levels: By targeting entry, which helps to improve oncotropism; and by targeting postentry sites after virus uptake, which improves the detargeting of native tissue for the enhancement of virus multiplication and expansion, including lysis of tumor cells.
Targeting tumor specific entry sites: The selectivity of tumours is an important feature of oncolytic viruses. Some OVs, such as reovirus, NDV, VSV (Vesicular Stomatitis Virus), parvovirus and bovine HSV-1, naturally have features of oncotropism [81-87]. Genetic engineering can be used to do this with different viruses. In naked viruses, targeting and detargeting are normally accomplished by modifying the capsid and, in enveloped viruses, by modifying the outer membrane [88]. Adaptor molecules have been used to conceal the capsid's cell-binding region, ligands have been inserted and capsid proteins (the cellular receptor’s target) have been genetically modified or inserted [89,90]. Particularly in cancers with diverse populations of tumor cells, a complete strategy should target tumor associated stromal components and tumor cells. This approach should also detarget healthy native tissues. The use of a complex mosaic technique (for example, hybrid fibres in adenovirus vectors) is an effective technique for expanding tropism by targeting a diverse population of tumor cells [91]. One of the uses of targeting entry and modification of the capsid is the creation of a transport method in which immunogenic proteins of the virus nucleo capsid are protected. These coverings lower the immunogenicity and inhibit viral breakdown, increasing viral efficiency. Modifying the surface epitopes of the virus can protect it against neutralising antibodies [92,93]. The novel viruses are chimaeras with unidentifiable serotypes that could be utilised in patients who are immune to previous viruses for sequential treatment. New serotypes’ introduction can affect or change the epitopes [94]. The insertion of a new serotype is done with just a single serotype; for example, in the MV, the glycoproteins are exchanged with the glycoproteins of the canine distemper virus [95]. For the treatment of cancer, there are still few limits on entrance targeting in the genetic engineering of OVs. In most cancers, there is no specific target on the cell surface that can be taken as a specific entrance gate for viruses. The introduction of ligands in viral capsids is difficult to execute in some viruses, and when it is accomplished, it disrupts the fusion of cells in enveloped viruses. While efforts to maintain cell fusion by altering envelope fusion proteins were successful, the resultant viruses were unable to produce sufficient particles [96].
Targeting post entry sites: An OV must have trait of tumor specific replication and must be unable to affect the normal cells. To achieve selective replication, various ways have been used. One method is to prevent the virus from reproducing in healthy cells by eliminating genes present in normal cells but deficient in tumor cells (like pathways for suppressing tumors); so, virus could only multiply in cells that have tumors. T-VEC, the only OV approved therapy of cancer yet, was found to have a therapeutic advantage in the phase III trial of OPTiM. The RL1 gene is removed in T-VEC (the backbone of HSV-1). Infected protein 34.5 is encoded by RL1. The interferon response suppresses viral replication in healthy cells, although protein 34.5 in infected cells acts as a viral replication rescuing mechanism. As a result, its absence prevents viral multiplication in normal cells. Tumor cells, on the other hand, are unaffected due to deficient response of interferon. ONYX015 is the 1st oncolytic adenovirus studied in medical testing. It is a virus in which the E1B55K gene, which typically binds and inactivates p53, was removed; consequently, the virus specifically duplicates in tumor cells with p53-mutations, ignoring healthy cells [97,98]. It was eventually reported that the mode of action was not always dependent on p53 and that the limitation of ONYX-015 in healthy cells was caused by the late export of viral RNA instead of the inactivation of p53 [99].
Another extensively employed therapeutic method is transcription targeting. The defined region of regulatory DNA that comprises a transcription start site is designed and assembled as a promoter segment for transcriptional targeting in viral cancer cell lysis. Regulatory components, including enhancers, binding sites of transcription factors, and rarely silencers, have also been coupled with the promoter fragment and substituted with viral promoter elements [100]. The very first research to reveal viral replication only in tumor cells after transcriptional targeting was the introduction of the prostate specific antigen promoter in the Ad E1A gene, which plays a significant role in Ad replication regulation. A follow up study found that adding an enhancer to the E1B gene increased specificity by 100-fold, demonstrating the efficiency of a multitarget strategy [101]. Tumour specific promoters are one of the most appealing classes of target for transcriptional targeting of different tumours, in contrast to promoters that are specific for tissues. The human telomerase reverse transcriptase promoter is predominantly activated in a wide range of cancers and it is widely used to produce a genetically engineered virus with a limited expression of E1A in tumor cells that is efficient against different cancerous cells and in preclinical models [102-104]. For example, some promoters like cyclooxygenase-2, Ki67, and L-plastin can control the replication of viruses in numerous malignancies [105-108]. Double targeting with promoters from both groups has also been used to improve therapeutic value in heterogeneous tumor cell populations, with significant improvements [109]. Thus, transcriptional targeting is an effective strategy for achieving virus replication in tumor cells that is extremely selective. Antitumor chemicals will have a more targeted effect on tumor cells when they are conjugated with oncolytic viruses.
Another important aspect of viral oncolysis is viral transmission. The extracellular matrix (which is mostly made up of collagen and hyaluronic acid) is a stromal factor that restricts oncolytic viruses from entering and spreading evenly throughout the tumor tissue. Various ways have been developed to promote viral dissemination and bypass the stromal barrier. One strategy is to use extracellular matrix-degrading proteins like relaxin. Relaxin has the unique property of inhibiting collagen generation while having no effect on basal collagen concentrations. In pancreatic cancer, relaxin-expressing oncolytic adenoviruses were demonstrated to break down the fibrotic extracellular matrix and improve tumor penetration and gemcitabine clinical efficacy [110]. Decorin down regulates and destroys extracellular matrix components in different ways, like by lowering collagen fibril thickness, blocking TGF beta, and boosting MMP 1 (matrix metalloproteinase 1) activity. Decorin-expressing oncolytic adenoviruses were created based on these modulatory properties, and in vivo investigations showed better tissue penetration and enhanced toxicity in cells with destruction of the desmoplastic extracellular matrix and suppression of epithelial to mesenchymal transitions [111,112].
Challenges for OVs in cancer treatment
Although oncolytic viruses offer potent pharmacological uses in malignancies, they still have several flaws that must be taken into consideration to enhance their efficiency in viral therapy. Virus administration methodologies, viral toxicity, and antiviral immunity in the body are all factors to consider. In solid tumours, oncolytic viruses must overcome a few challenges to reach the tumour's core. The physical impediment could be a substantial impediment in the virus delivery process because the virus needs to cross the endothelium layer to get to its destination [113]. Aside from the endothelium layer barriers, excessive vascular permeability, an aberrant lymphatic system within tumours and interstitial hypertension generated by the extracellular matrix of solid tumours could all impede virus entry [114]. Another major impediment to oncolytic viruses is the huge number of single barriers found in the immunosuppressive microenvironment of solid tumours [115]. When tumor cells are attached to TME, they resist immune surveillance, multiply quickly and spread. Tumour Associated Macrophages (TAM), neutrophils, Tumor Infiltrating T Lymphocytes (TIL) and Tumor Associated Fibroblasts (TAF) are all recruited by solid tumours by secreting cytokines and chemokines [116]. These cells may be able to protect tumours from immune responses with properly functioning anticancer cells. In tumor infiltrating T cells, this causes overexpression of suppression signals and immune regulatory point receptors, leading to the formation of an immunosuppressive tumor microenvironment [114-117]. Furthermore, low pH micro environmental therapy and post-local hypoxia can prevent tumor cell apoptosis, boost angiogenesis, increase factors that help in tumor growth, and turn cancer cells resilient to traditional treatments like chemotherapeutics and radiation [118,119]. As a result, once the oncolytic viruses enter the tumor area, they must continue to play a part in the immunosuppressive tumor microenvironment.
Results and Discussion
Use of combinatorial therapy
Combinatorial therapy is a type of therapy that combines
two or more therapies. With recent advancements in cancer
immunotherapy methods, the concept of combinatorial
therapy with immunotherapy and oncolytic viruses
became an interesting option. Researchers have tried
combining oncolytic virotherapy with other anti tumor
therapies involving chemotherapy, CAR-T cell therapies,
radiotherapy, Immune Checkpoint Inhibitors (ICIs) and bi
specific T cell engagers. Such combinatorial therapies with
oncolytic viruses could be useful for further enhancing
treatment consequences as multiple component strategies
can report the errors of every component.
OVs and chemotherapy/radiotherapy: For the treatment
of cancer, chemotherapy and radiotherapy are being used
either in combination or alone. For localised control of
tumours, radiotherapy is used, and it results in various
antitumor effects [120]. In preclinical models, radiotherapy
and OVs are used in combination because OVs alone
have limited success. When used in combination with
radiotherapy, oncolytic VACV, VSV, adenovirus, and
HSV show therapeutic benefits [121-123]. Strong anti
tumor results are shown when radiotherapy is applied
in conjunction with OV and this approach is effective
against those tumours that are unable to be cured by other
therapies [124]. In Diffuse Intrinsic Pontine Gliomas
(DIPGs) and pHGG (Paediatric High Grade Gliomas)
models, OV Delta-24-RGD was tested along with
radiotherapy [125]. Results indicated that OV reduced the
repair proteins for DNA damage, enhanced immune cell
trafficking, sensitised tumor cells to the consequences of
radiotherapy and increased mouse survival. Therefore, OVmediated
obstruction of pathways for cellular DNA repair
can sensitise tumor cells to radiotherapy. In the same way,
when oncolytic VSV expressing IFN (VSV-IFN) is used
along with radiotherapy, the anticancer response of the
immune system is enhanced and the tumor in syngeneic
models is reduced [126].
At present, phase I trials in clinics for localised advancedstage
rectal cancer with chimeric adenovirus type 11p and
chemotherapy, radiotherapy and chemoradiotherapy are in
progress (NCT03916510). For enhancing OV therapeutic
effects, OVs were tested in combination with conventional
chemotherapeutics. The main objective was to decrease the
toxic outcomes of drugs while increasing OVs efficiency
in the TME (tumor microenvironment). But, in a few
preclinical tumor models, certain drugs behaved as anti
virals and decreased the replication of viruses in tumor
beds depending on chemotherapeutic drug type and dose
schedules [127,128]. The studies directed clinical trials on
numerous aggressive tumor types like pancreatic, ovarian,
breast, brain, melanoma and myeloma with OVs and
standard chemotherapeutic drugs (cyclophosphamide,
paclitaxel, gemcitabine, cisplatin, doxorubicin, doxycycline
and temozolomide) [129].
OVs and immune checkpoint inhibitors: ICIs (Immune
Checkpoint Inhibitors), which attack checkpoints like PDL1,
PD-1, or CTLA-4 and damage the ability of cancer cells
to elude the immune response of the host, have emerged as
important cancer treatment options. Nonetheless, despite
their many successes, ICIs have a few serious limitations:
• Even with tumours that are appropriately targeted,
only a small percentage of patients (almost 20 percent)
respond to ICI treatment.
• Some patients undergoing ICI therapy have
experienced immune-related side effects.
• Immunologically "cold" tumours with a minimal TIL
(tumor-infiltrating lymphocyte) count have a limited
impact.
In this situation, oncolytic viruses, specifically genetically
altered oncolytic viruses, can boost infiltration and
activation of lymphocytes and disrupt cancer cells. To
address the ineffectiveness of ICIs in many patients,
oncolytic virotherapy coupled with ICIs has been
performed in animal models and medical trials with
encouraging results [130,131]. A lot of oncolytic viruses
like VACV (Vaccinia virus), NDV (Newcastle disease
virus), adenovirus, HSV (herpes simplex virus), and
VSV (vesicular stomatitis virus) are now being tested in
conjunction with ICIs in research trials. Recent research
suggests that, in conjunction with ICIs, genetically altered
OVs and OVs producing immune-activating cytokines
with increased ability to change TME have shown improved
efficiency. Immunotherapy, for instance, improved the
antitumor action of a recombinant orthopoxvirus (CF33)
and a live attenuated ZIKV vaccine candidate [132,133].
Ribas A et al reported a phase 1b clinical trial examining the
effects of talimogene laherparepvec oncolytic virotherapy on cytotoxic T cell infiltration and the therapeutic potency
of pembrolizumab, an anti PD-1 antibody. Twenty-one
patients with metastatic melanoma received talimogene
laherparepvec treatment before receiving pembrolizumab
as part of a combo therapy. The majority of adverse effects
were mild, with weariness, fevers and chills being the
most frequent ones. No toxicities with dosage limitations
happened. After receiving talimogene laherparepvec,
patients who responded to combination therapy exhibited
higher levels of CD8+ T cells, PD-L1 protein expression and
IFN-gamma gene expression on a number of cell subsets in
tumors. It didn't seem that the response to combination
therapy was related to the baseline CD8+ T cell infiltration
or the baseline IFN-gamma signature [134]. Chesney J,
et al. evaluated the efficacy of talimogene laherparepvec
and ipilimumab in combination vs. ipilimumab alone in
patients with advanced melanoma in a phase II research.
In order to evaluate the results of pairing a checkpoint
inhibitor with an oncolytic virus, this was the first
randomised experiment. Responses were not limited to
injected lesions; 23% of patients receiving ipilimumab and
52% of individuals receiving the combination experienced
decreases in visceral lesions. They arrived to the conclusion
that the combination has better anticancer activity
than ipilimumab without creating any additional safety
concerns [135]. The novel class of immunotherapeutics
known as Bispecific T Cell Engagers (BiTEs) directs T
cells to tumor surface antigens. While effective against
some haematological malignancies, widespread clinical
application has been hindered thus far, particularly
against solid tumours, by poor bioavailability and severe
toxicities. Oncolytic Viruses (OVs) are a new type of cancer
immunotherapy that selectively infect and proliferate in
cancerous cells, mediating the effects of tumor vaccination.
These oncotropic viruses can work in conjunction with
other immunotherapies and act as carriers for tumor
targeted immunomodulation. Heidbuechel and Engeland
studied the design and properties of the OV-BiTE vector
as well as the proof of immune stimulating and anti tumor
activities. Additionally, they cover various CAR T cell and
immune checkpoint inhibitor combo regimens as well
as OV-BiTE based techniques for modulating the tumor
microenvironment [136]. These innovative medicines'
inherent complexity emphasises the value of translational
research, which includes correlative investigations in early-stage
clinical trials. Broadly speaking, OV-BiTEs can act
as a model for various OV-based cancer immunotherapies.
Oncolytic virotherapy and cell therapy: Adoptive cell
therapy (cellular immunotherapy) eliminates cancer cells
by using modified copies of immune system cells. Cellular
immunotherapies of different kinds have now been
established, including TCR (engineered T Cell Receptor)
therapy, NK (Natural Killer) cell therapy, CAR (Chimeric
Antigen Receptor) T cell therapy and TIL (Tumor
Infiltrating Lymphocyte) therapy. In all these treatments,
CAR-T cell therapy has displayed extraordinary success for
blood cancer as a promising immunotherapy. Moreover, due
to a deficiency of infiltration and continuous presence in the
tissues of the tumor, cell treatment has a low success rate,
especially in solid tumours. Cell treatment in combination
with oncolytic viruses has been explored to address
these restrictions. In this scenario, the use of genetically
altered oncolytic viruses exhibiting medicinal transgenes
can improve the therapeutic efficacy of both therapeutic
approaches. For instance, B7H3-targeted CAR-T used
in conjunction with an oncolytic adenovirus expressing
IL-7 demonstrated greater efficacy as compared to single
therapy alone [137]. T cell expansion was increased, and
T cell apoptosis was inhibited when combined with OV.
Cell treatment could be used in conjunction with multi-armed
OVs in the future. For instance, HER2-specific
CAR T cells were employed along with adenovirus-based
OVs expressing a BiTE molecule, checkpoint blocking and
cytokines to greatly increase tumor control and survival
[138].
OVs and bispecific T cell engagers: BiTEs (Bispecific T
Cell Engagers) are bispecific antibodies that use a peptide
linker to connect two scFvs (Single Chain Variable
Fragments). BiTE single chain variable fragments have
two arms: 1 arm binds with CD3 or activators of T cells
that are present on their surface, while the second arm
attaches with a target antigen that is present on the surfaces
of cancer cells. The attachment of both arms with their
respective target antigens activates T lymphocytes, which
causes disruption of target tumor cells. In serum, the half-life
of BiTE molecules is very short and shows poor tumor
penetration. The BiTE molecule also exhibits dose limiting
toxicities [139]. As a result, developing OVs that encode
BiTEs is a useful technique for addressing these issues. The
oncolytic vaccinia virus designed to target the EphA2-TEA-VV
(Tumor Cell Surface Antigen Ephrin A2) was used to
create the first OV containing BiTE. When applied in
conjunction with peripheral blood mononuclear cells from
humans, EphA2-TEA-VV has strong antitumor effects in
a preclinical lung cancer xenograft model, according to Yu,
et al. [140]. Fajardo, et al. created ICOVIR-15K-cBiTE by
modifying an oncolytic adenovirus called ICOVIR-15K to
produce an EGFR-targeting BiTE (cBiTE) antibody [141].
Activating T cell receptor signaling while targeting
signaling antigens with bi and tri specific antibodies
has also shown considerable potential in cancer
immunotherapy. Researchers have developed a cutting-edge
method for combining the two distinct anti-cancer
techniques, turning them into OVs equipped with bi or
tri specific T cell engagers (BiTE or TriTE) for tailored
immunotherapy. This combinatorial approach has been
investigated by numerous research teams since 2014 and it
demonstrated significant efficacy in a range of tumor types.
The development of T Cell Receptor Mimics (TCRm)
into BiTEs is anticipated to significantly increase the use
of BiTEs and BiTE-armed OVs for the efficient targeting
of intracellular tumor antigens by Guo ZS, et al [142].
Clinical investigations are actively testing oncolytic vectors
from various viral families. Majority of OV-BiTE papers
examined adenovirus based oncolytics, which is in line
with the present state of clinical virotherapy trials [143].
An interim futility analysis led to the early termination of enrollment in the phase III clinical study for the
treatment of hepatocellular cancer for JX-594 or PexaVec,
a VV encoding GM-CSF (NCT02562755). Systemic anti
tumor immunity can be produced by polyclonal T cells
through priming, activation, proliferation, trafficking,
memory formation, cytokine release, and cytotoxic action
as a result of OV-mediated APC maturation and antigen
cross-presentation.
Oncolytic viruses and autophagy: Autophagy plays role
in breakdown of cellular components. When autophagy
interacts with immunological activities, it assists the body
in fighting infection and removing foreign invaders [144].
Viruses engage with the autophagy apparatus of a host
during their life span, with either a positive or harmful
effect for themselves, according to mounting evidence
[145,146]. In this context, oncolytic viral infection
regulates autophagy, and the viruses' impacts on cellular
autophagy are virus-specific and a little bit complicated.
Interplay among autophagy and oncolytic viruses in cancer
treatment is summarized in Fig. 3.
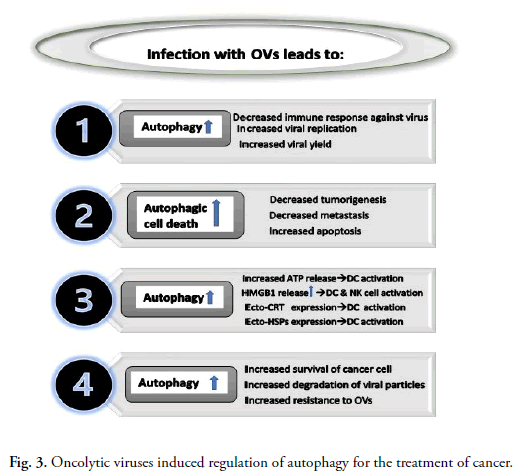
Fig 3. Oncolytic viruses induced regulation of autophagy for the treatment of cancer.
Induction of autophagic cell death: Autophagic cell
death is sort of controlled cell death which is dependent
on autophagic apparatus [147]. Apart from its role in
infectivity and replication of OVs, autophagy also plays
an important role in oncolytic virus-induced cancer cell
death. OBP-301, for instance, causes cell death induced
by autophagy via the E2F1-miR-7-EGFR pathway. Cell
survival is inhibited by upregulated miR7 and this miR7
initiates autophagy by negatively regulating the EGFR
(epidermal growth factor receptor) [147]. By arming
oncolytic viruses with Beclin 1, one can improve their
therapeutic effectiveness by eliciting autophagic cell death.
A chimeric oncolytic Ad SG511-BECN (with Beclin 1) was
engineered by Tong, et al. and they tested its efficacy against
cancer. They discovered that therapy with SG511-BECN
caused substantial cell death stimulated by autophagy
in the cells of leukaemia, as well as increased survival
and reduced tumor size in leukaemia bearing animals
[148]. In addition, coupling SG511-BECN with the
chemotherapeutic drug doxorubicin can boost the virus's
pathogenic capacity [149]. Squamous cell carcinoma,
when infected with HSV-1 RH2 (deficient in 34.5 genes),
causes LC3 accumulation in cytoplasm, transition of
LC3-I to LC3-II, the production of autophagosomes,
and autophagic cell death, according to Furukawa et al.
Although inhibiting autophagy had no impact on virus
multiplication, it significantly reduced RH2 cytotoxicity
[150]. According to Zhang, et al., when a cell gets infected
with an oncolytic virus, it induces autophagic cell death,
which can ultimately prevent carcinogenesis [151].
Regulation of immunogenic cell death: Autophagy
influences the survivability, multiplication, differentiation
and stimulation of immune system components like T and
B lymphocytes, NK (Natural Killer) cells, macrophages
and DCs (dendritic cells), which can impact innate and
adaptive immune reactions [152]. On the other side, some
immune-related cells, immunoglobulins and cytokines
have a significant impact on autophagy activity. TGF
(Transforming Growth Factor), Interferon Gamma,
Interleukin 12 (IL-12), IL-1 and IL-2 induce autophagy
activity, whereas interleukin 4, interleukin-10 and
interleukin-13 inhibit the function of autophagy in the
body [153].
In oncolytic virotherapy, autophagy has been reported to
induce Immunogenic Cell Death (ICD). Immunogenic
cell death is described as the process in which intracellular
components, including DAMPs, PAMPs, and TAAs, cause
an antitumor immune response [154]. Such immunogenic
substances can cause dendritic cells to become activated,
and T-cells are then exposed to antigens via these cells.
Liikanen, et al., for instance, examined the effectiveness of
oncolytic adenovirus in conjunction with Temozolomide
(TMZ), as well as the impact on autophagy and
immunological reactions. Furthermore, they discovered
that combining the therapeutic effect of temozolomide
with oncolytic Ad5/3-D24-GM-CSF decreased tumor
development, increased autophagy, triggered immunogenic
cell death by increasing CRT (calreticulin), HMGB1 (High
Mobility Group Box-1) expression and ATP secretion
[155]. Calreticulin (an Endoplasmic Reticulum-linked
chaperon) moves towards the plasma membrane's external
layer in dying cells (ecto CRT) [156]. The representation
of calreticulin on cancerous cells in the process of cell
death leads to the ingestion of cancerous cell materials by
dendritic cells in addition to the presentation of tumor-specific
antigens. These antigens then promote CTL
(cytotoxic T lymphocyte) specific responses in the body
[157]. Moreover, ecto-calreticulin-positive cells increase
the expression levels of IL-6 and TNF on DCs (dendritic
cells), causing pro-inflammatory Th17 (T helper type 17)
to become polarised [158]. Adenosine triphosphate that
gets expelled by dead cells interacts with receptors like
metabotropic (P2Y2) and ionotropic (P2X7), which are
present on APC and increases their chemotactic attraction
and maturation [159-161]. APCs triggered by ATP secrete
IL-1, which increases the formation of IFN by cytotoxic
T lymphocytes (CTLs) [162]. After infection, cancerous
cells passively secrete HMGB1 in the nucleus [163]. The
release of HMGB1 has been linked to the activation of
autophagy in anti tumor therapy [164]. In an extracellular
environment, HMGB1 binds to TLR4 (Toll Like Receptor
4) and stimulates dendritic cells, which in turn activate
CTLs [165]. According to another study, glioma cells were
infected with an oncolytic NDV (New Castle disease virus),
which stimulated autophagy and Immunogenic Cell Death
(ICD). ICD induction was associated with increased cell
surface CRT, PMEL17 and HMGB1 secretion. Intra
tumoral delivery of NDV to glioma bearing mice causes
high-level production of IFN-gama and CD8+/CD4+ T
cells in microenvironment of tumor, as determined by in
vivo characterization of immunogenic cell death. It also
causes a decrease in the fraction of suppressor cells that
were derived from myeloid cells [166]. After infecting
with oncolytic NDV, autophagy-dependent immunogenic
cell death was experiential in lungs cancerous cells, as
evidenced by elevated levels of HSP70/90, HMGB1 and
ATP, along with increased expression of calreticulin [167].
Ecto-HSP70 and 90 have been reported to bind with
receptors present on APC surface to induce activation of
CD8+ T-cell [168]. They could stimulate dendritic cells
development activation of NK cells, generation of pro-inflammatory
cytokines [169-171]. Conversely, NDV/FMW elevated the level of ICD markers in cancer cells,
while autophagy suppression causes reduced FMW/NDV-induced
ICD marker secretion, like HSP70/90 (heat shock
proteins) and HMGB1. Furthermore, STAT3 inhibition
also reduces the NDV/FMW-induced ICD within cancer
cells [172]. FMW/NDV may promote immunogenic
cell death independent of the autophagy process because
cytoplasmic STAT3 inhibits eIF2A phosphorylation by
interacting with the PKR kinase and inhibiting autophagy
[173].
Conclusion
OVs specifically replicate in cancerous cells and destroy
these defective cells. Their specificity and toxicity could
be greatly increased by genetic engineering techniques.
Oncolytic virotherapy, like some other cancer therapeutic
medications, has various drawbacks, including antiviral
immunological reactions, tumor mass invasion and virus
delivery to the destination. In this article, we've covered
several different combinatorial tactics with oncolytic
viruses to increase anti tumor immunity and maintain their
cytotoxic action against a tumor in the immunosuppressive
tumor microenvironment. Most of the researchers have
tried oncolytic virotherapy in conjunction with other
standard treatments like chemotherapy, radiotherapy, etc.,
or cancer immune therapies such as inhibitors for immune
checkpoint and CAR-T therapy. Viruses interact with the
autophagy apparatus of the host and assist the body in
fighting infection. Other issues in oncolytic virotherapy
include the duration and pattern of administration of
autophagy enhancers, which can improve their anti tumor
actions by activating oncolysis. Many more studies are
required to entirely understand how they interact and how
they can be used together in cancer treatment.
Conflict of Interest
There is no conflict of interest in this review article.
Funding Agency
There is no funding agency for this study.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-10.
[Crossref] [Google Scholar] [PubMed]
- Chakraborty S, Rahman T. The difficulties in cancer treatment. Ecancermedicalscience 2012;6: ed16.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist 2019;2: 141-0.
[Crossref] [Google Scholar] [PubMed]
- Gujar S, Pol JG, Kroemer G. Heating it up: Oncolytic viruses make tumors ‘hot’and suitable for checkpoint blockade immunotherapies. Oncoimmunology 2018;7: e1442169.
[Crossref] [Google Scholar] [PubMed]
- Coley WB. Contribution to the knowledge of sarcoma. Ann Surg 1891;14: 199-220.
[Crossref] [Google Scholar] [PubMed]
- Ma XY, Hill BD, Hoang T, et al. Virus-inspired strategies for cancer therapy. Semin Cancer Biol 2022;86: 1143-7
[Crossref] [Google Scholar] [PubMed]
- Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci 2016;107: 1373-9.
[Crossref] [Google Scholar] [PubMed]
- Goldufsky J, Sivendran S, Harcharik S, et al. Oncolytic virus therapy for cancer. Oncolytic Virother 2013;23: 31-6.
[Crossref] [Google Scholar] [PubMed]
- Thorne SH, Hermiston T, Kirn D. Oncolytic virotherapy: Approaches to tumor targeting and enhancing antitumor effects. Semin Oncol 2005;32: 537-8.
[Crossref] [Google Scholar] [PubMed]
- Duncan MR, Stanish SM, Cox DC. Differential sensitivity of normal and transformed human cells to reovirus infection. J Virol 1978;28: 444-9.
[Crossref] [Google Scholar] [PubMed]
- Strong JE, Coffey MC, Tang D, et al. The molecular basis of viral oncolysis: Usurpation of the Ras signaling pathway by reovirus. EMBO J 1998;17: 3351-2.
[Crossref] [Google Scholar] [PubMed]
- Coffey MC, Strong JE, Forsyth PA, et al. Reovirus therapy of tumors with activated Ras pathway. Science 1998;282: 1332-4.
[Crossref] [Google Scholar] [PubMed]
- Gong J, Sachdev E, Mita AC, et al. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J Methodol 2016;6: 25.
[Crossref] [Google Scholar] [PubMed]
- Morris DG, Feng X, DiFrancesco LM, et al. REO-001: A phase I trial of percutaneous intralesional administration of reovirus type 3 dearing (Reolysin®) in patients with advanced solid tumors. Invest New Drugs 2013;31: 696-706.
[Crossref] [Google Scholar] [PubMed]
- Kicielinski KP, Chiocca EA, John SY, et al. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther 2014;22: 1056-2.
[Crossref] [Google Scholar] [PubMed]
- Galanis E, Markovic SN, Suman VJ, et al. Phase II trial of intravenous administration of Reolysin®(Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther 2012;20: 1998-3.
[Crossref] [Google Scholar] [PubMed]
- Dorig RE, Marcil A, Chopra A, et al. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993;75: 295-305.
[Crossref] [Google Scholar] [PubMed]
- Tatsuo H, Ono N, Tanaka K, et al. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000;406: 893-7.
[Crossref] [Google Scholar] [PubMed]
- Anderson BD, Nakamura T, Russell SJ, et al. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer res 2004;64: 4919-6.
[Crossref] [Google Scholar] [PubMed]
- Kurokawa C, Iankov ID, Anderson SK, et al. Constitutive interferon pathway activation in tumors as an efficacy determinant following oncolytic virotherapy. J Natl Cancer Inst 2018;110: 1123-2.
[Crossref] [Google Scholar] [PubMed]
- Achard C, Boisgerault N, Delaunay T, et al. Sensitivity of human pleural mesothelioma to oncolytic measles virus depends on defects of the type I interferon response. Oncotarget 2015;6: 44892-4.
[Crossref] [Google Scholar] [PubMed]
- Aref S, Bailey K, Fielding A. Measles to the rescue: A review of oncolytic measles virus. Viruses 2016;8: 294.
[Crossref] [Google Scholar]
- Lawler SE, Speranza MC, Cho CF, et al. Oncolytic viruses in cancer treatment: A review. JAMA Oncol 2017;3: 841-9.
[Crossref] [Google Scholar] [PubMed]
- Msaouel P, Opyrchal M, Dispenzieri A, et al. Clinical trials with oncolytic measles virus: Current status and future prospects. Curr Cancer Drug Targets 2018;18: 177-87.
[Crossref] [Google Scholar] [PubMed]
- Simmonds P, Gorbalenya AE, Harvala H, et al. Recommendations for the nomenclature of enteroviruses and rhinoviruses. Arch Virol 2020;165: 793-7.
[Google Scholar]
- Donina S, Strele I, Proboka G, et al. Adapted ECHO-7 virus Rigvir immunotherapy (oncolytic virotherapy) prolongs survival in melanoma patients after surgical excision of the tumour in a retrospective study. Melanoma Res 2015;25: 421-6.
[Crossref] [Google Scholar] [PubMed]
- Malogolovkin A, Gasanov N, Egorov A, et al. Combinatorial approaches for cancer treatment using oncolytic viruses: Projecting the perspectives through clinical trials outcomes. Viruses 2021;13: 1271.
[Crossref] [Google Scholar] [PubMed]
- Holl EK, Brown MC, Boczkowski D, et al. Recombinant oncolytic poliovirus, PVSRIPO, has potent cytotoxic and innate inflammatory effects, mediating therapy in human breast and prostate cancer xenograft models. Oncotarget. 2016;7: 79828.
[Crossref] [Google Scholar] [PubMed]
- Ochiai H, Campbell SA, Archer GE, et al. Targeted therapy for glioblastoma multiforme neoplastic meningitis with intrathecal delivery of an oncolytic recombinant poliovirus. Clin Cancer Res 2006;12: 1349-54.
[Crossref] [Google Scholar] [PubMed]
- Annels NE, Mansfield D, Arif M, et al. Phase I trial of an ICAM-1-targeted immunotherapeutic-coxsackievirus A21 (CVA21) as an oncolytic agent against non muscle-invasive bladder cancer. Clin Cancer Res 2019;25: 5818-31.
[Crossref] [Google Scholar] [PubMed]
- Hamid O, Ismail R, Puzanov I. Intratumoral immunotherapy-update 2019. Oncologist 2020;25:3-8.
[Crossref] [Google Scholar] [PubMed]
- Bradley S, Jakes AD, Harrington K, et al. Applications of coxsackievirus A21 in oncology. Oncolytic Virother 2014: 7-5.
[Crossref] [Google Scholar] [PubMed]
- Au GG, Beagley LG, Haley ES, et al. Oncolysis of malignant human melanoma tumors by Coxsackieviruses A13, A15 and A18. Virol J 2011;8: 1-6.
[Crossref] [Google Scholar] [PubMed]
- Hwang JK, Hong J, Yun CO. Oncolytic viruses and immune checkpoint inhibitors: Preclinical developments to clinical trials. Int J Mol Sci 2020;21: 8627.
[Crossref] [Google Scholar] [PubMed]
- Bodian D. Histopathologic basis of clinical findings in poliomyelitis. Am J Med 1949;6: 563-78.
[Crossref] [Google Scholar] [PubMed]
- Desjardins A, Gromeier M, Herndon JE, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 2018;379: 150-61.
[Crossref] [Google Scholar] [PubMed]
- Taylor MW, Cordell B, Souhrada M, Prather S. Viruses as an aid to cancer therapy: Regression of solid and ascites tumors in rodents after treatment with bovine enterovirus. Proc Natl Acad Sci USA 1971;68: 836-40.
[Crossref] [Google Scholar] [PubMed]
- Zheltukhin AO, Soboleva AV, Sosnovtseva AO, et al. Human enteroviruses exhibit selective oncolytic activity in the model of human glioblastoma multiforme xenografts in immunodeficient mice. Bulletin of RSMU. 2018: 42-8.
[Google Scholar]
- Hoos A. Development of immuno-oncology drugs-from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov 2016;15: 235-47.
[Crossref] [Google Scholar] [PubMed]
- Podshivalova ES, Semkina AS, Kravchenko DS, et al. Efficient delivery of oncolytic enterovirus by carrier cell line NK-92. Mol Ther Oncolytics 2021;21: 0-8.
[Crossref] [Google Scholar] [PubMed]
- Alberts P, Tilgase A, Rasa A, et al. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur J Pharmacol 2018;837: 117-26.
[Crossref] [Google Scholar] [PubMed]
- Beasley GM, Nair SK, Farrow NE, et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J Immunother Cancer 2021;9: 002203.
[Crossref] [Google Scholar] [PubMed]
- Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: A new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14: 642-62.
[Crossref] [Google Scholar] [PubMed]
- Elankumaran S, Chavan V, Qiao D, et al. Type I interferon-sensitive recombinant newcastle disease virus for oncolytic virotherapy. J Virol 2010;84: 3835-44.
[Crossref] [Google Scholar] [PubMed]
- Schirrmacher V. Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance. Expert Opin Biol Ther 2015;15: 1757-71.
[Crossref] [Google Scholar] [PubMed]
- Matveeva OV, Guo ZS, Senin VM, et al. Oncolysis by paramyxoviruses: Preclinical and clinical studies. Mol Ther Oncolytics 2015;150017.
[Crossref] [Google Scholar] [PubMed]
- Csatary LK, Gosztonyi G, Szeberenyi J, et al. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J Neurooncol 2004;67: 83-3.
[Crossref] [Google Scholar] [PubMed]
- Integrative PD. Newcastle Disease Virus (PDQ®). InPDQ Cancer Information Summaries. National Cancer Institute (US). 2018.
[Google Scholar]
- Taguchi S, Fukuhara H, Homma Y, et al. Current status of clinical trials assessing oncolytic virus therapy for urological cancers. Int J Urol 2017;24(5): 342-351.
[Crossref] [Google Scholar] [PubMed]
- Molouki A, Peeters B. Rescue of recombinant Newcastle disease virus: Current cloning strategies and RNA polymerase provision systems. Arch Virol 2017;162: 1-12.
[Crossref] [Google Scholar] [PubMed]
- Molouki A, Peeters B. Rescue of recombinant Newcastle disease virus: A short history of how it all started. Arch Virol 2017;162: 5-4.
[Crossref] [Google Scholar] [PubMed]
- Goradel NH, Mohajel N, Malekshahi ZV, et al. Oncolytic adenovirus: A tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol 2019;234: 8636-46.
[Crossref] [Google Scholar] [PubMed]
- Alemany R. Oncolytic adenoviruses in cancer treatment. Biomedicines 2014;2: 36-49.
[Crossref] [Google Scholar] [PubMed]
- Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr Cancer Drug Targets 2018;18: 171-6.
[Crossref] [Google Scholar] [PubMed]
- Russell L, Peng KW. The emerging role of oncolytic virus therapy against cancer. Chin Clin Oncol 2018;7: 16.
[Crossref] [Google Scholar] [PubMed]
- Heise C, Hermiston T, Johnson L, et al. An adenovirus E1A mutant that demonstrates potent and selective systemic anti-tumoral efficacy. Nat Med 2000;6: 1134-9.
[Crossref] [Google Scholar] [PubMed]
- Fueyo J, Gomez-Manzano C, Alemany R, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000 ;19: 2-12.
[Crossref] [Google Scholar] [PubMed]
- Philbrick B, Adamson DC. DNX-2401: An investigational drug for the treatment of recurrent glioblastoma. Expert Opin Investig Drugs 2019;28: 1-9.
[Crossref] [Google Scholar] [PubMed]
- Kuryk L, Moller AS, Jaderberg M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 2019;8: e1532763.
[Crossref] [Google Scholar] [PubMed]
- Hemminki A. Oncolytic immunotherapy: Where are we clinically? Scientifica 2014;2014: 862925.
[Crossref] [Google Scholar] [PubMed]
- Peters C, Rabkin SD. Designing herpes viruses as oncolytics. Mol Ther Oncolytics 2015;2: 15010.
[Crossref] [Google Scholar] [PubMed]
- Kohlhapp FJ, Kaufman HL. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res 2016;22: 1048-54.
[Crossref] [Google Scholar] [PubMed]
- Conry RM, Westbrook B, McKee S, et al. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum Vaccin Immunother 2018;14: 839-46.
[Crossref] [Google Scholar] [PubMed]
- Tomazin R, van Schoot NE, Goldsmith K, et al. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol 1998;72: 2560-3.
[Crossref] [Google Scholar] [PubMed]
- Eissa IR, Naoe Y, Bustos-Villalobos I, et al. Genomic signature of the natural oncolytic herpes simplex virus HF10 and its therapeutic role in preclinical and clinical trials. Front Oncol 2017;7: 149.
[Crossref] [Google Scholar] [PubMed]
- Hirooka Y, Kasuya H, Ishikawa T, et al. A Phase I clinical trial of EUS-guided intratumoral injection of the oncolytic virus, HF10 for unresectable locally advanced pancreatic cancer. BMC Cancer 2018;18: 596.
[Crossref] [Google Scholar] [PubMed]
- Breitbach CJ, Bell JC, Hwang TH, et al. The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594). Oncolytic Virother 2015;28: 25-31.
[Crossref] [Google Scholar] [PubMed]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100: 57-70.
[Crossref] [Google Scholar]
- Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: A phase I trial. Lancet Oncol 2008;9: 533-42.
[Crossref] [Google Scholar] [PubMed]
- Smith GL, Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene 1983;25: 21-8.
[Crossref] [Google Scholar] [PubMed]
- Puhlmann M, Brown CK, Gnant M, et al. Vaccinia as a vector for tumor-directed gene therapy: Biodistribution of a thymidine kinase-deleted mutant. Cancer Gene Ther 2000;7: 66-73.
[Crossref] [Google Scholar] [PubMed]
- Thorne SH, Hwang TH, O’Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest 2007;117: 3350-8.
[Crossref] [Google Scholar]
- McCart, JA, Ward JM, Lee J, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 2001;61: 8751-7.
[PubMed]
- Shi T, Song X, Wang Y, et al. Combining oncolytic viruses with cancer immunotherapy: Establishing a new generation of cancer treatment. Front Immunol 2020;11: 683.
[Crossref] [Google Scholar] [PubMed]
- Johansson ES, Xing L, Cheng RH, et al. Enhanced cellular receptor usage by a bioselected variant of coxsackievirus a21. J Virol 2004;78: 12603-12.
[Crossref] [Google Scholar] [PubMed]
- Dorer DE, Nettelbeck DM. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 2009;61: 554-71.
[Crossref] [Google Scholar] [PubMed]
- Guo ZS, Thorne SH, Bartlett DL. Oncolytic virotherapy: Molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim Biophys Acta 2008;1785: 217-31.
[Crossref] [Google Scholar] [PubMed]
- Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 2000;6: 821-5.
[Crossref] [Google Scholar] [PubMed]
- Fernandes J. Oncogenes: The passport for viral oncolysis through PKR inhibition. Biomark Cancer 2016;8:BIC-S33378.
[Crossref] [Google Scholar] [PubMed]
- Escamilla-Tilch M, Filio-Rodriguez G, Garcia-Rocha R, et al. The interplay between pathogen-associated and danger-associated molecular patterns: An inflammatory code in cancer. Immunol Cell Biol 2013;91: 601-10.
[Crossref] [Google Scholar] [PubMed]
- Abd-Aziz N, Poh CL. Development of oncolytic viruses for cancer therapy. Transl Res 2021;237:98-123.
[Crossref] [Google Scholar] [PubMed]
- Buijs PR, Van Eijck CH, Hofland LJ, et al. Different responses of human pancreatic adenocarcinoma cell lines to oncolytic Newcastle disease virus infection. Cancer Gene Ther 2014;21: 24-30.
[Crossref] [Google Scholar] [PubMed]
- Parato, KA, Senger D, Forsyth PA, et al., Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005;5: 965-76.
[Crossref] [PubMed]
- Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther 2002;9: 961-6.
[Crossref] [Google Scholar] [PubMed]
- Cornelis JJ, Lang SI, Stroh-Dege AY, et al. Cancer gene therapy through autonomous parvovirus-mediated gene transfer. Curr Gene Ther 2004;4:249-61.
[Crossref] [Google Scholar] [PubMed]
- Cuddington BP, Dyer AL, Workenhe ST, et al. Oncolytic bovine herpesvirus type 1 infects and kills breast tumor cells and breast cancer-initiating cells irrespective of tumor subtype. Cancer Gene Ther 2013;20: 282-9.
[Crossref] [Google Scholar] [PubMed]
- Maroun J, Munoz-Alia M, Ammayappan A, et al. Designing and building oncolytic viruses. Future Virol 2017;12: 193-213.
[Crossref] [Google Scholar] [PubMed]
- Haisma HJ, Grill J, Curiel DT, et al. Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther 2000;7: 901-4.
[Crossref] [Google Scholar] [PubMed]
- Krasnykh V, Belousova N, Korokhov N, et al. Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol 2001;75: 4176-83.
[Crossref] [Google Scholar] [PubMed]
- Hesse A, Kosmides D, Kontermann RE, et al. Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J Virol 2007 Mar 15;81(6): 2688-99.
[Crossref] [Google Scholar] [PubMed]
- Kaliberov SA. Experimental virotherapy of chemoresistant pancreatic carcinoma using infectivity-enhanced fiber-mosaic oncolytic adenovirus. Cancer Gene Ther 2014;21:264-74.
[Crossref] [Google Scholar] [PubMed]
- Kambara H. An oncolytic HSV-1 mutant expressing ICP34. 5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res 2005;65:2832-9.
[Crossref] [Google Scholar] [PubMed]
- Deng L, Fan J, Ding Y, et al. Oncolytic efficacy of thymidine kinase deleted vaccinia virus strain Guang9. Oncotarget 2017;8:40533-43.
[Crossref] [Google Scholar] [PubMed]
- Kaufmann JK, Nettelbeck DM. Virus chimeras for gene therapy, vaccination, and oncolysis: Adenoviruses and beyond. Trends Mol Med 2012;18:365-76.
[Crossref] [Google Scholar] [PubMed]
- Tatsuo H, Yanagi Y. The morbillivirus receptor SLAM (CD150). Microbiol Immunol 2002;46:135-42.
[Crossref] [Google Scholar] [PubMed]
- Hudacek W, Navaratnarajah CK, Cattaneo R. Development of measles virus-based shielded oncolytic vectors: Suitability of other paramyxovirus glycoproteins. Cancer Gene Ther 2013;20: 109-16.
[Crossref] [Google Scholar] [PubMed]
- Nemunaitis J, Khuri F, Ganly I. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol 2001;19: 289-98.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Hong J, Oh JE, et al. Potent antitumor effect of tumor microenvironment-targeted oncolytic adenovirus against desmoplastic pancreatic cancer. Int J Cancer 2018;142: 392-413.
[Crossref] [Google Scholar] [PubMed]
- O'Shea CC, Johnson L, Bagus B, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer cell 2004;6: 611-23.
[Crossref] [Google Scholar] [PubMed]
- Terai K, Bi D, Liu Z, et al. A novel oncolytic herpes capable of cell-specific transcriptional targeting of CD133 ± cancer cells induces significant tumor regression. Stem Cells 2018;36: 1154-69.
[Crossref] [Google Scholar] [PubMed]
- Moaven O. Strategies to develop potent oncolytic viruses and enhance their therapeutic efficacy. JCO Precis Oncol 2021;5: 733-43.
[Crossref] [Google Scholar] [PubMed]
- Watanabe Y, Hashimoto Y, Kagawa S, et al. Enhanced antitumor efficacy of telomerase-specific oncolytic adenovirus with valproic acid against human cancer cells. Cancer Gene Ther 2012;19: 767-72.
[Crossref] [Google Scholar] [PubMed]
- Lin WH, Yeh SH, Yang WJ, et al. Telomerase specific oncolytic adenoviral therapy for orthotopic hepatocellular carcinoma in HBx transgenic mice. Int J Cancer 2013;132: 1451-62.
[Crossref] [Google Scholar] [PubMed]
- Tazawa H, Hasei J, Yano S, et al. Bone and soft-tissue sarcoma: a new target for telomerase-specific oncolytic virotherapy. Cancers 2020;12: 478.
[Crossref] [Google Scholar] [PubMed]
- Nakagawa T, Tanaka H, Shirakawa T, et al. Cyclooxygenase 2 promoter based replication selective adenoviral vector for hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg 2009;135: 282-6.
[Crossref] [Google Scholar] [PubMed]
- Bauerschmitz GJ, Guse K, Kanerva A, et al. Triple targeted oncolytic adenoviruses featuring the cox2 promoter, E1A transcomplementation, and serotype chimerism for enhanced selectivity for ovarian cancer cells. Mol Ther 2006;14: 164-74.
[Crossref] [Google Scholar] [PubMed]
- Akbulut H, Zhang L, Tang Y, et al. Cytotoxic effect of replication-competent adenoviral vectors carrying L-plastin promoter regulated E1A and cytosine deaminase genes in cancers of the breast, ovary and colon. Cancer Gene Ther 2003;10: 388-95.
[Crossref] [Google Scholar] [PubMed]
- Hoffmann D, Meyer B, Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. J Gene Med 2007;9: 764-78.
[Crossref] [Google Scholar] [PubMed]
- Doloff JC, Waxman DJ. Dual E1A oncolytic adenovirus: Targeting tumor heterogeneity with two independent cancer-specific promoter elements, DF3/MUC1 and hTERT. Cancer gene ther 2011;18: 153-66.
[Crossref] [Google Scholar] [PubMed]
- Jung KH, Choi IK, Lee HS, et al. Oncolytic adenovirus expressing relaxin (YDC002) enhances therapeutic efficacy of gemcitabine against pancreatic cancer. Cancer lett 2017;396: 155-66.
[Crossref] [Google Scholar] [PubMed]
- Choi I, Lee YS, Yoo JY, et al. Effect of decorin on overcoming the extracellular matrix barrier for oncolytic virotherapy. Gene ther 2010;17: 190-201.
[Crossref] [Google Scholar] [PubMed]
- Li Y, Hong J, Jung BK, et al. Oncolytic Ad co-expressing decorin and Wnt decoy receptor overcomes chemoresistance of desmoplastic tumor through degradation of ECM and inhibition of EMT. Cancer lett 2019;459: 15-29.
[Crossref] [Google Scholar] [PubMed]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 2010;7: 653-64.
[Crossref] [Google Scholar] [PubMed]
- Kuczynski EA, et al. Vessel co-option in cancer. Nat Rev Clin Oncol 2019;16: 469-93.
[Crossref] [Google Scholar] [PubMed]
- Sharp DW, Lattime EC. Recombinant poxvirus and the tumor microenvironment: oncolysis, immune regulation and immunization. Biomedicines 2016;4: 19.
[Crossref] [Google Scholar] [PubMed]
- Guedan S, Alemany R. CAR-T cells and oncolytic viruses: Joining forces to overcome the solid tumor challenge. Front Immunol 2018;9: 2460.
[Crossref] [Google Scholar] [PubMed]
- Sobhanimonfared F, et al. Virus specific tolerance enhanced efficacy of cancer immuno-virotherapy. Microb Pathog 2020;140: 103957.
[Crossref] [Google Scholar] [PubMed]
- Oh E, Hong J, Kwon OJ, et al. A hypoxia and telomerase responsive oncolytic adenovirus expressing secretable trimeric TRAIL triggers tumour specific apoptosis and promotes viral dispersion in TRAIL resistant glioblastoma. Sci Rep 2018;8: 1420.
[Crossref] [Google Scholar] [PubMed]
- Vaupel P. The role of hypoxia induced factors in tumor progression. Oncologist 2004;9:10-7.
[Crossref] [Google Scholar] [PubMed]
- Chicas-Sett R, Zafra-Martin J, Morales-Orue I, et al. Immunoradiotherapy as an effective therapeutic strategy in lung cancer: From palliative care to curative intent. Cancers 2020;12: 2178.
[Crossref] [Google Scholar] [PubMed]
- Waters AM, Johnston JM, Reddy AT, et al. Rationale and design of a phase 1 clinical trial to evaluate HSV G207 alone or with a single radiation dose in children with progressive or recurrent malignant supratentorial brain tumors. Hum Gene Ther Clin Dev 2017;28: 7-16.
[Crossref] [Google Scholar] [PubMed]
- Mell LK, Brumund KT, Daniels GA, et al. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin Cancer Res 2017;23: 5696-702.
[Crossref] [Google Scholar] [PubMed]
- O’Cathail SM, Pokrovska TD, Maughan TS, et al. Combining oncolytic adenovirus with radiation—a paradigm for the future of radiosensitization. Front Oncol 2017;7: 153.
[Crossref] [Google Scholar] [PubMed]
- Simbawa E, Al-Johani N, Al-Tuwairqi S. Modeling the spatiotemporal dynamics of oncolytic viruses and radiotherapy as a treatment for cancer. Comput Math Methods Med 2020;2020: 3642654.
[Crossref] [Google Scholar] [PubMed]
- Martinez-Velez N, Marigil M, Garcia-Moure M, et al. Delta-24-RGD combined with radiotherapy exerts a potent antitumor effect in diffuse intrinsic pontine glioma and pediatric high grade glioma models. Acta Neuropathol Commun 2019;7: 1-2.
[Crossref] [Google Scholar] [PubMed]
- Udayakumar TS, Betancourt DM, Ahmad A, et al. Radiation attenuates prostate tumor antiviral responses to vesicular stomatitis virus containing IFNβ, resulting in pronounced antitumor systemic immune responses. Mol Cancer Res 2020;18: 1232-43.
[Crossref] [Google Scholar] [PubMed]
- Kellish P, Shabashvili D, Rahman MM, et al. Oncolytic virotherapy for small-cell lung cancer induces immune infiltration and prolongs survival. J Clin Invest 2019;129: 2279-92.
[Crossref] [Google Scholar] [PubMed]
- Wennier ST, Liu J, Li S, et al. Myxoma virus sensitizes cancer cells to gemcitabine and is an effective oncolytic virotherapeutic in models of disseminated pancreatic cancer. Mol Ther 2012;20: 759-68.
[Crossref] [Google Scholar] [PubMed]
- Malfitano AM, Di Somma S, Iannuzzi CA, et al. Virotherapy: From single agents to combinatorial treatments. Biochem Pharmacol 2020;177: 113986.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Li Y, Chen K, et al. Oncolytic virotherapy reverses the immunosuppressive tumor microenvironment and its potential in combination with immunotherapy. Cancer Cell Int 2021;21: 262.
[Crossref] [Google Scholar] [PubMed]
- LaRocca CJ, Warner SG. Oncolytic viruses and checkpoint inhibitors: Combination therapy in clinical trials. Clin Transl Med 2018;7: 35.
[Crossref] [Google Scholar] [PubMed]
- Kim SI, Park AK, Chaurasiya S, et al. Recombinant orthopoxvirus primes colon cancer for checkpoint inhibitor and cross-primes T cells for antitumor and antiviral immunity. Mol Cancer Ther 2021;20: 173-82.
[Crossref] [Google Scholar] [PubMed]
- Nair S, Mazzoccoli L, Jash A, et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. J CIin insight 2021;6: 144619
[Crossref] [Google Scholar] [PubMed]
- Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves anti-PD-1 immunotherapy. Cell 2017;170:1109-19.
[Crossref] [Google Scholar] [PubMed]
- Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol 2018;36: 1658.
[Crossref] [Google Scholar]
- Heidbuechel JP, Engeland CE. Oncolytic viruses encoding bispecific T cell engagers: a blueprint for emerging immunovirotherapies. J Hematol Oncol 2021;14: 63.
[Crossref] [Google Scholar] [PubMed]
- Huang J, Zheng M, Zhang Z, et al. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol Immunother 2021;70: 2453-5.
[Crossref] [Google Scholar] [PubMed]
- Porter CE, Shaw AR, Jung Y, et al. Oncolytic adenovirus armed with BiTE, cytokine and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol Ther 2020;28: 1251-62.
[Crossref] [Google Scholar] [PubMed]
- Scott EM, Duffy MR, Freedman JD, et al. Solid tumor immunotherapy with T cell engager-armed oncolytic viruses. Macromol Biosci 2018;18: 1700187.
[Crossref] [Google Scholar] [PubMed]
- Yu F, Wang X, Guo ZS, et al. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol Ther 2014;22: 102-11.
[Crossref] [Google Scholar] [PubMed]
- Fajardo CA, Guedan S, Rojas LA, et al. Oncolytic adenoviral delivery of an EGFR-targeting T-cell engager improves antitumor efficacy. Cancer Res 2017;77: 2052-63.
[Crossref] [Google Scholar] [PubMed]
- Guo ZS, Lotze MT, Zhu Z, et al. Bi-and tri-specific T cell engager-armed oncolytic viruses: Next-generation cancer immunotherapy. Biomedicines 2020;8: 204.
[Crossref] [Google Scholar] [PubMed]
- Macedo N, Miller DM, Haq R, et al. Clinical landscape of oncolytic virus research in 2020. J Immunother Cancer 2020;8: e001486
[Crossref] [Google Scholar] [PubMed]
- Kuballa P, Nolte WM, Castoreno AB, et al. Autophagy and the immune system. Annu Rev Immunol 2012;30: 611-46.
[Crossref] [Google Scholar] [PubMed]
- Sumpter, Jr R, Levine B. Selective autophagy and viruses. Autophagy 2011;7: 260-5.
[Crossref] [Google Scholar] [PubMed]
- Kudchodkar SB, Levine B. Viruses and autophagy. Rev Med Virol 2009;19: 359-78.
[Crossref] [Google Scholar] [PubMed]
- Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018;25: 486-541.
[Crossref] [Google Scholar]
- Tong Y, You L, Liu H, et al. Potent antitumor activity of oncolytic adenovirus expressing Beclin-1 via induction of autophagic cell death in leukemia. Oncotarget 2013;4: 860-74.
[Crossref] [Google Scholar] [PubMed]
- Li L, You LS, Mao LP, et al. Combing oncolytic adenovirus expressing Beclin-1 with chemotherapy agent doxorubicin synergistically enhances cytotoxicity in human CML cells in vitro. Acta Pharmacol Sin 2018;39: 251-60.
[Crossref] [Google Scholar] [PubMed]
- Furukawa Y, Takasu A, Yura Y. Role of autophagy in oncolytic herpes simplex virus type 1-induced cell death in squamous cell carcinoma cells. Cancer Gene Ther 2017;24: 393-400.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Lai W, Li Q, et al. A novel oncolytic adenovirus targeting Wnt signaling effectively inhibits cancer-stem like cell growth via metastasis, apoptosis and autophagy in HCC models. Biochem Biophys Res Commun 2017;491: 469-77.
[Crossref] [Google Scholar] [PubMed]
- Jiang X, Wang J, Deng X, et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol Cancer 2019;18:1-7.
[Crossref] [Google Scholar] [PubMed]
- Harris J Autophagy and cytokines. Cytokine 2011;56: 140-4.
[Crossref] [Google Scholar] [PubMed]
- Serrano-del Valle A, Anel A, Naval J, et al. Immunogenic cell death and immunotherapy of multiple myeloma. Front Cell Dev Biol 2019;7: 50.
[Crossref] [Google Scholar] [PubMed]
- Liikanen I, Ahtiainen L, Hirvinen ML, et al. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther 2013;21:1212-23.
[Crossref] [Google Scholar]
- Inoue H, Tani KJ. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ 2014;21: 39-9.
[Crossref] [Google Scholar] [PubMed]
- Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31: 51-72.
[Crossref] [Google Scholar]
- Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun 2011;2: 521.
[Crossref] [Google Scholar] [PubMed]
- Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009;461: 282-6.
[Crossref] [Google Scholar] [PubMed]
- Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nat Med 2009;15: 1170-8.
[Crossref] [Google Scholar] [PubMed]
- Galluzzi L, Pietrocola F, Bravo-San Pedro JM, et al. Autophagy in malignant transformation and cancer progression. EMBO J 2015;34: 856-80.
[Crossref] [Google Scholar] [PubMed]
- Hou W, Zhang Q, Yan Z, et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 2013;4: e966.
[Crossref] [Google Scholar] [PubMed]
- Garg AD, Nowis D, Golab J, et al. Immunogenic cell death, DAMPs and anticancer therapeutics: An emerging amalgamation. Biochim Biophys Acta 2010;1805: 53-71.
[Crossref] [Google Scholar] [PubMed]
- Thorburn J, Horita H, Redzic J, et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ 2009;16: 175-83.
[Crossref] [Google Scholar] [PubMed]
- Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007;220: 47-59.
[Crossref] [Google Scholar] [PubMed]
- Koks CA, Garg AD, Ehrhardt M, et al. Newcastle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death. Int J Cancer 2015;136: E313-25.
[Crossref] [Google Scholar] [PubMed]
- Ye T, Jiang K, Wei L, et al. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells. Am J Cancer Res 2018;8: 1514-27.
[Google Scholar] [PubMed]
- Panzarini E, Inguscio V, Dini L. Immunogenic cell death: Can it be exploited in photo dynamic therapy for cancer. Biomed Res Int 2013;2013: 482160.
[Crossref] [Google Scholar] [PubMed]
- Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96–associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med 2000;191:1965-4.
[Crossref] [Google Scholar] [PubMed]
- Hickman-Miller HD, Hildebrand WH. The immune response under stress: The role of HSP-derived peptides. Trends in immunol 2004;25: 427-3.
[Crossref] [Google Scholar]
- Lehner T, Wang Y, Whittall T, et al. Functional domains of HSP70 stimulates generation of cytokines and chemokines, maturation of dendritic cells and adjuvanticity. Biochem Soc Trans 2004;32: 629-2.
[Crossref] [Google Scholar] [PubMed]
- Shao X, Wang X, Guo X, et al. STAT3 contributes to oncolytic newcastle disease virus-induced immunogenic cell death in melanoma cells. Front Oncol 2019;9: 436.
[Crossref] [Google Scholar] [PubMed]
- Jonchere B, Belanger A, Guette C, et al. STAT3 as a new autophagy regulator. Jak Stat 2013;2: 667-0.
[Crossref] [Google Scholar] [PubMed]








