Keywords
|
| |
| Plastic membrane; Coated wire electrode; Ion-selective electrode; Tetrazepam; Potentiometric determination. |
| |
Introduction
|
| |
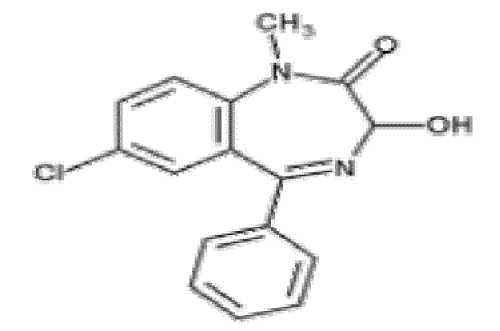
| Tetrazepam, 7-chloro-5 (cyclohex - 1enyl) - 1methyl – 1,3-dihydro-2H-1,benzodiazepin-2-one, the chemical structure was depicted in Fig.1. Tetrazepam, is a benzodiazepine derivative with anxiolytic and muscle relaxant properties. It is used mainly to treat anxiety disorders such as panic attacks, or more rarely to treat depression, premenstrual syndrome or agoraphobia. Tetrazepam has relatively little sedative effect at low doses while still producing useful muscle relaxation and anxiety relief [1]. |
| |
| Several methods for its determination have been reported, both in body fluids and pharmaceuticals, including high performance liquid chromatography[2-4], liquid chromatography coupled with mass spectrometry [5,6]. This work describes a new selective membrane sensors, of two types: plastic membrane and coated wire electrodes for the determination of tetrazepam in pure solutions, pharmaceutical preparations and biological fluids. |
| |
| Several methods for its determination have been reported, both in body fluids and pharmaceuticals, including high performance liquid chromatography[2-4], liquid chromatography coupled with mass spectrometry [5,6]. This work describes a new selective membrane sensors, of two types: plastic membrane and coated wire electrodes for the determination of tetrazepam in pure solutions, pharmaceutical preparations and biological fluids. |
| |
|
Experimental
|
| |
|
Standard Drug Solution
|
| |
| Stock tetrazepam solution (1x10-1M) was prepared daily by dissolving an appropriate amount of the drug in acetonitrile. More dilute solutions were prepared by appropriate dilution using double distilled water. |
| |
| Recommended procedures |
| |
| Preparation of Tetrazepam-Phosphomolybdate Ionpair: |
| |
| The ion-pair was prepared by mixing stoichiometric amounts of 1x10-2M phosphomolybdic acid with an equimolar solution of tetrazepam, stirred for 10 min. The resulting yellowish precipitate was filtered through G4 sintered glass crucible and washed thoroughly with deionized water then dried at room temperature for 24 hours. The ion-pair should be stored in a desiccator. |
| |
| Membrane Composition: |
| |
| The membrane composition was studied by varying the percentages (w/w) of the ion pair, poly(vinyl chloride) PVC and plasticizer onitrophenyl octyl ether, until the optimum composition that exhibits the best performance characteristics was obtained. the membranes were prepared by dissolving the required amount of the ion-pair, PVC and (o-NPOE), in 5 mL tetrahydrofuran (THF). The solution mixture was poured into a petri dish (3 cm in diameter), covered with a filter paper and the solvent was allowed to evaporate slowly at room temperature. To obtain the uniform membrane thickness, the amount of (THF) was kept constant, and its evaporation was fixed for 24h. |
| |
| Electrode Construction: |
| |
| Plastic membrane electrode: A punched circular membrane was attached to a poly-ethylene tube (8 mm in diameter) in an electrode configuration by means of PVC-THF solution. A mixture containing equal volume of 1x10-3M tetrazepam and potassium chloride was used as internal reference solution in which the Ag/AgCl reference electrode was dipped. The constructed electrode was pre-conditioned after preparation by soaking for 5 h in 1x10-3M tetrazepam and stored in the same solution. All potentiometric measurements were performed using the following cell assembly: Ag/AgCl/Internal solution /membrane/test solution//KCl salt bridge//SCE. |
| |
| Coated wire electrode: Pure aluminum wire of 4.0 cm length was tightly insulated by polyethylene tube leaving 1.0 cm at one end for the coating and 0.5 cm at the other end for connection. The coating solution was (described previously under membrane composition). Prior to coating, the polished aluminum surface was washed with a detergent and water, thoroughly rinsed with water, and dried with acetone. Then the wire was rinsed with chloroform and allowed to dry. Afterwards, the aluminum wire was coated by quickly dipping it into the coating solution several times, and allowing the film left on the wire to dry for about 3 min. The process was repeated several times until a plastic membrane of approximately 1.0 mm thickness was formed. The prepared electrode was conditioned by soaking for 3 h in 1.0x10-3 M tetrazepam solution. All potentiometric measurements were performed using the following cell assembly: Al/membrane/test solution//KCl salt bridge//SCE. |
| |
|
Determination of tetrazepam in dosage forms
|
| |
| Tetrazepam Tablets |
| |
| Ten tablets were finely powdered, shaken with 25 mL acetonitrile, distilled water was used for serial dilution to obtain different concentrations in the range of 5x10-3-1x10-5M, of tetrazepam for both electrode I and II. The prepared solutions were adjusted to pH 4 using 0.1N dilute hydrochloric acid. The tetrazepam-electrode(s) was immersed in the solution. The electrode(s) system was allowed to equilibrate with stirring and the e.m.f. recorded and compared the calibration graph. The standard addition (spiking technique) was also applied by recording the electrode(s) potential after the addition of 0.1 mL of standard 1x10-1M tetrazepam solution to the above drug test solutions. |
| |
| Content Uniformity Assay of Tetrazepam Tablets |
| |
| Ten individual tablets of tetrazepam 50 mg/tablet were placed in separate 100 mL beakers and dissolved in 10 mL acetonitrile, complete 90-100 mL of distilled water. The electrode(s) was directly immersed into 10 mL of each sample for five times and should be washed with deionized water to reach steady potential between the individual measurements. The mean potential was used to evaluate the content uniformity from the calibration graph. |
| |
|
Application to serum and urine
|
| |
| Adjust urine pH to 5 (using 0.1N hydrochloric acid) and pH of serum to 6 (use phosphate buffer). Add hydrochloric acid to urine and phosphate buffer to serum dropwise until the suitable pH obtained. Transfer 5 mL previously adjusted urine or serum into small separatory funnels, and then separately add 5 mL, 10-3, 10-4 and 10-5, 10-6 M standard drug solution, followed by the addition of 20 mL toluene for urine or 20 mL diethyl ether for serum. Shake each funnel for 5 min, and transfer aqueous layer to centrifuge tube. Centrifuge for 2 min at 1500 rpm, transfer each solution to a 50 mL volumetric flask, and dilute to volume with deionized water. Apply above procedure as described under electrode calibration [7]. |
| |
Results and discussion
|
| |
| Optimization of Membrane Composition |
| |
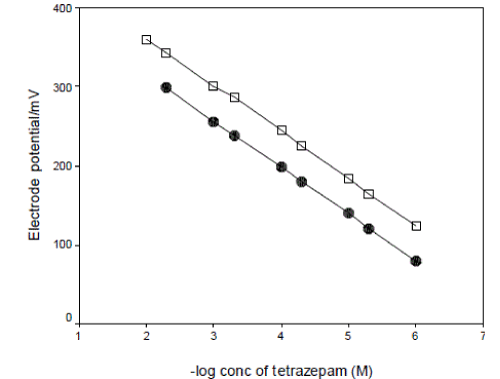
| In the previous experimental investigations[8], It is obvious that both kind of plasticizer selected and the membrane composition used can influence the response performances (such as the sensitivity, linear concentration range, the detection limit, the response time etc.) of PVC membrane sensors, if other properties of the sensor, e.g. selectivity or pH response, are omitted. In this study four membrane compositions were investigated, the results were summarized in Table 1. The results showed that the electrode(s) made by membrane of type (d) with 3.0 wt% tetrazepam-phosphomolybdate ion pair, 30.0 wt% PVC and 67.0 wt% plasticizer o-NPOE exhibits the best performance characteristics (slope 58.88±0.5 and 59.18±0.1 mV decade-1 at 25°C for electrode I and II respectively, over tetrazepam concentration range from 5x10-3-1x10-6M and 1x10- 2-1x10-6M for electrode I and II respectively. |
| |
| Nature and Response Characteristics of The Electrode(s) |
| |
| Tetrazepam reacts with phosphomolybdic acid to form a stable tetrazepam-phosphomolybdate ionpair complex which is water insoluble but readily soluble in an organic solvent such as tetrahydrofuran. The complex was prepared and tested as active material with o-NPOE as a solvent mediator in a poly (vinyl chloride) membrane response for tetrazepam. The critical response characteristics of plastic membrane and coated wire electrodes were determined and results are summarized in Table 2. The electrode(s) exhibits a Nernstain response over the concentration range from 5x10-3-1x10-6M and 1x10-2-1x10-6M tetrazepam for electrode I and II respectively, with a slope 58.88±0.5 and 59.18±0.1 mV decade-1 change in concentration for electrode I and II respectively as in Fig. 2. The choice of membrane solvent to achieve the required selectivity is based on its electric permittivity and its immiscibility with aqueous phase, high viscosity, low solubility of the matrix in the membrane and ability to dissolve ionpair complex. |
| |
| Life time |
| |
| The response time of the electrode(s) was tested for 1x10-1-1x10-6 M tetrazepam solutions. The sequence of measurements was from low to high concentrations. The electrode(s) exhibits a fast dynamic response of 15, 10 s, for electrode I and II respectively. The electrode(s) used for a period of 20 and 27 days for electrode I and II respectively, without significant change in the electrode(s) parameters. |
| |
| Effect of Plasticizer |
| |
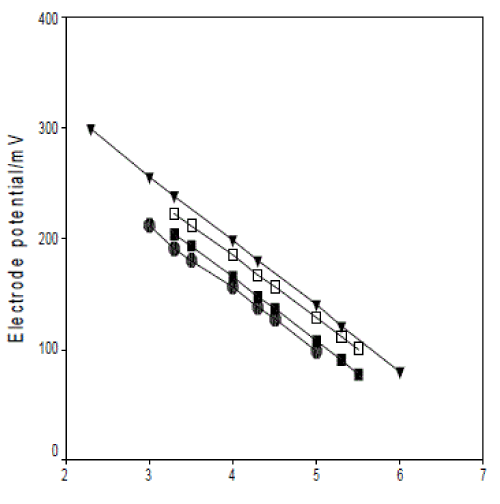
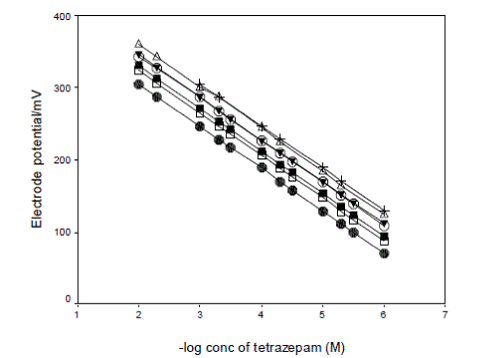
| In this study, four plasticizers, di-butyl sebacate (DBS), di-butylphthalate (DBP) and dioctylphthalate (DOP) and o-nitrophenyl octyl ether (o-NPOE) were used to examine the optimization of the membrane with plasticizer entailed the use of plasticizer content ratio, 58.0, 62.0, 64.0 and 67.0 wt%, and the use of PVC contents of 40.0, 37.0, 34.5, and 30.0 wt%. The electroactive compound (tetrazepam-phosphomolybdate) contents of 2.0, 1.0, 1.5 and 3.0 wt%. The results obtained showed that the response performances of the prepared membranes were rather different depending on the use of plasticizer, the proportion of the plasticizer toward PVC and of the electroactive compound. The typical potential responses of the electrodes constructed with four plasticizers were given in Fig. 3. As shown in Fig. 3, the o-NPOE-PVC electrodes were superior to DBS- , DBP- , DOPPVC electrodes in both the response slope and linear concentration range. So o-NPOE was selected as the plasticizer of the membranes. The best membrane composition of the o-NPOE-PVC electrode(s) was 30.0 wt % PVC, 67.0 wt % o-NPOE and 3.0 wt % ion-pair. |
| |
| Effect of Soaking |
| |
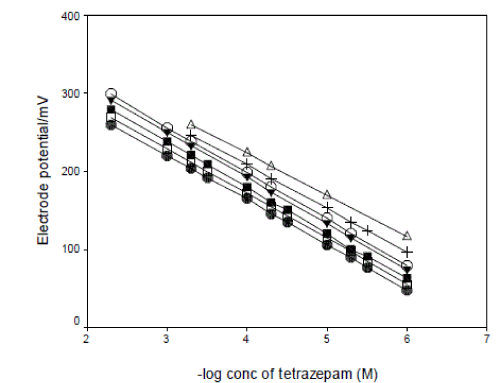
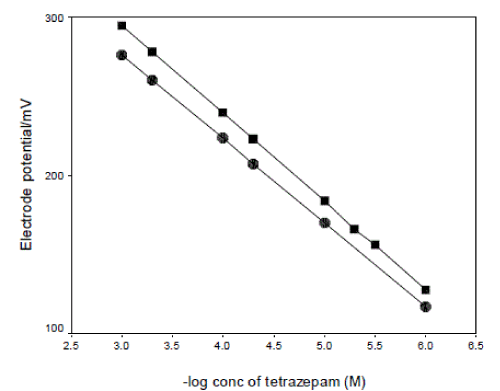
| The performance characteristics of the tetrazepamphosphomolybdate electrode(s) was studied as a function of soaking time. For this purpose the electrode(s) was soaked in 1x10-3M solution of tetrazepam and the calibration graphs were plotted after 1, 2, 3, and 24h. the optimum soaking time was found to be 5 h, 3 h at which the slope of the calibration curve was 58.88±0.5, 59.18±0.1 mV decade-1, at 25 ºC for electrode I and II respectively. The influence of prolonged soaking on the lifetime of tetrazepam-phosphomolybdate electrode(s) was followed by constructing calibration plots. The electrode(s) was soaked continuously on 1x10-3M solution of tetrazepam for 24 h, 7, 10, 15, 20, 27 and 30 days. The calibration plot slopes decreased slightly to 52.96, 57.99 mV decade-1 after 20 and 27 days for electrode I and II respectively. Fig. 4, 5, show the effect of prolonged soaking time and the life span of the tetrazepam-phosphomolybdate electrodes. |
| |
| Regeneration of the Electrode |
| |
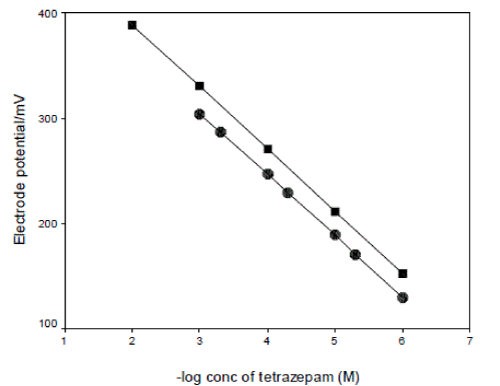
| The above discussion reveals that soaking of the electrode(s) in the drug solution for a long time has a negative effect on the response of the membrane towards the tetrazepam. The same effect appears after working with the electrode(s) for a long time. The regeneration of the electrode(s) was tried simply by reformation of the ion-exchange on the external gel layer of membrane [9]. The regeneration of the tetrazepam-phosphomolybdate membrane was successfully achieved by soaking the exhausted electrode(s) for 24 h in a solution that was 1x10-2M phosphomolybdic acid, followed by soaking for 3 h in 1x10-2M tetrazepam solution. Fig. 6, 7, show the calibration graphs for an exhausted electrode(s) (slopes 52.96, 57.99 mV decade-1) for electrode I and II respectively, and for the same electrode(s) after regeneration (slopes 55.74, 58.90 mV decade-1) for electrode I and II respectively. It was found that the lifespan of the regenerated electrode(s) is limited to 3 h due to the ease of leaching of the lipophilic salts from the gel layer at the electrode(s) surface compared with those that are attached homogeneously to the PVC network through the solvent mediator. |
| |
| Effect of pH |
| |
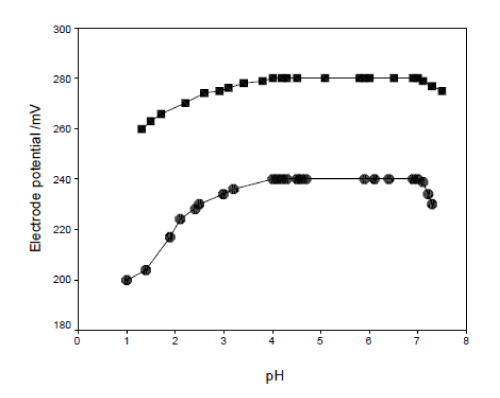
| The effect of pH of the tetrazepam solution using 1x10-3 M tetrazepam on the electrode(s) potential was investigated. The solution was acidified by the addition of very small volumes of hydrochloric acid 0.1N then the pH value was increased gradually using sodium hydroxide 0.1N for each pH value, the potential was recorded and thus the potential-pH curves for tetrazepam concentration were constructed as in Fig. 8. As is obvious, within the pH range 4-6 for both electrodes. The electrode(s) potential is practically independent of pH, and in this range the electrode(s) can be safely used for tetrazepam determination. |
| |
| Selectivity of the electrode |
| |
| The influence of various basic substances on the response of tetrazepam sensors was investigated by measuring the potentiometric interference from many sugars, inorganic cations, certain alkaloids and amino acids. The selectivity coefficients were evaluated by the separate solution method. Table 3, showed that the proposed tetrazepamphosphomolybdate membrane electrode(s) is highly selective toward tetrazepam. The electrode(s) showed no response to a number of potentially interfering ionic excipients usually used in the manufacturing of the pharmaceutical preparations, such as starch and lactose. The inorganic cations did not interfere due to the differences in their mobilities and permiabilities as compared with tetrazepam cation. In the case of amino acids, the high selectivity is mainly attributed to the difference in polarity and lipophilic character of their molecules relative to tetrazepam. |
| |
| Quantification of tetrazepam: |
| |
| Direct potentiometric determination of tetrazepam using tetrazepam-phosphomolybdate electrode(s) type I and II was performed and calculated from the calibration curve. The direct potentiometric determination of tetrazepam in pure form using the proposed electrodes gave average recovery % of 99.56±0.4 and 99.59±0.5 for electrode I and II respectively. Furthermore, the results obtained were compared with the official method [10], (potentiometric titration using 0.1 M perchloric acid, for determination of tetrazepam), and the results are listed in Table 4. |
| |
|
Validation of the proposed ISE method
|
| |
| Accuracy |
| |
| The accuracy of the proposed ISE method was investigated by the determination of tetrazepam in spiked placebo samples prepared from serial concentrations of tetrazepam reference standards. The results summarized in Table 5, show that the proposed ISE method is an accurate one for the determination of tetrazepam in their pharmaceutical preparations without interfering from the coformulated adjuvants as indicated by the percentage recovery values. |
| |
| Linearity |
| |
| Under the optimal experimental ISE conditions, linear relationships exist between the electrode potential/mV and the logarithm of corresponding concentration of the investigated drug. The regression data, correlation coefficients (r) and other statistical parameter are previously listed in Table 2. |
| |
| Precision |
| |
| The precision of the proposed ISE method, measured as percentage relative standard deviation (RDS%) was tested by repeating the proposed method for determination of the investigated drug in its pharmaceutical preparations “ three batches” to nine replicates. The RSD% values for the repeated determinations were 0.480 %, 0.686% and 0.393% for determination of tetrazepam in Myolastan® tablets using tetrazepam-phosphomolybdate plastic membrane electrode and 0.728 %, 0.601 % and 0.426% in Myolastan® tablets using electrode tetrazepam-phosphotungstate coated wire electrode . The above RSD% values are less than 2% indicating good precision. |
| |
| Robustness and Ruggedness |
| |
| The robustness of the proposed ISE method was tested by investigating the capacity of the method to remain unaffected by a small but a deliberate variation in method parameters and provide an indication of its reliability during normal usage [11]. While the ruggedness of the proposed method was investigating the degree of reproducibility at test results obtained by the analysis of the same samples under a variety of conditions such as different laboratories, analysts and instruments. The results obtained by using another model of pH-meter (Orion 420 A) were compared with those obtained using model of pH-meter (Jenway 3040). The obtained results are close and also reveal validity of the method. The results previously listed in Table 2. |
| |
| Detection limit |
| |
| The detection limit of the investigated drug was calculated according to IUPAC recommendation which stated that the detection limit is the concentration at which the measured potential differs from that predicted by the linear regression by more than 18 mV. The values were previously reported in Table 2, indicate that the proposed ISE method is sensitive for detection of very small concentrations of tetrazepam. |
| |
|
Analytical Applications of the proposed method
|
| |
| Application to pharmaceutical preparations Tetrazepam Tablets: |
| |
| The proposed ISE method was applied to determination of tetrazepam in their dosage forms. The mean % recovery found and RSD%, indicate that the proposed validated method could be adopted for the determination of the investigated drug in its pharmaceutical preparations without interference from the coformulated adjuvants. Table 6, shows the results obtained from the determination of tetrazepam in tablets in comparison with official method [10]. |
| |
| Content Uniformity Assay of Myolastan® Tablets |
| |
| The proposed ISE method described good accuracy and precise for the quality control tests, the content uniformity assay showed that the (R.S.D < 2%), with mean standard deviation 99.28±0.9 and 99.46±0.5 for electrode I ,and II respectively. |
| |
| Application to serum and urine |
| |
| The proposed ISE method was applied to determination of tetrazepam in biological fluids as human serum and urine. The results obtained were summarized in Table 7. |
| |
Conclusion
|
| |
| The tetrazepam-selective electrodes based on tetrazepam-phosphomolybdate ion association in a PVC matrix exhibited useful analytical characteristics for the determination of tetrazepam in pure form, in pharmaceutical formulations and biological fluids. The good recoveries and low relative standard deviation reflect the high accuracy and precision of the proposed method. Moreover, the method is simple, easy to operate and in expensive making it an excellent tool for the routine determination of tetrazepam in quality control laboratories. |
| |
Source of Support
|
| |
| NIL |
| |
Conflict of Interest
|
| |
| NONE |
| |
Tables at a glance
|
 |
|
 |
|
 |
|
 |
| Table 1 |
|
Table 2 |
|
Table 3 |
|
Table 4 |
 |
|
 |
|
 |
| Table 5 |
|
Table 6 |
|
Table 7 |
|
| |
Figures at a glance
|
 |
|
 |
|
 |
|
 |
| Figure 1 |
|
Figure 2 |
|
Figure 3 |
|
Figure 4 |
 |
|
 |
|
 |
|
 |
| Figure 5 |
|
Figure 6 |
|
Figure 7 |
|
Figure 8 |
|
| |














