Keywords
ATF4; Quercetin; Novel object recognition; Alzheimer’s disease
Abbreviations
ATF4: Activating Transcription Factor 4; APP: Amyloid-β Precursor Protein; CREB: Cyclic Adenosine Monophosphate Response Element Binding Protein; eIF2α: Eukaryotic Translation Initiation Factor 2α; ISR: Integrated Stress Response
Introduction
Activating transcription factor 4 (ATF4) is translated at low levels in the presence of the eukaryotic translation initiation factor 2α (eIF2α)-GTP-tRNAiMet ternary complex [1] when eIF2α is phosphorylated at serine 51. The eIF2α protein can be phosphorylated by four mammalian eIF2α kinases, namely, protein kinase RNA (PKR)-like ER-localized eIF2α kinase (PERK), general control non-derepressible 2 (GCN2), PKR, and hemeregulated inhibitor [2]. In contrast, in the presence of the catalytic subunit of protein phosphatase 1 (PP1c)-binding protein (PPP1R15A), which is also known as growth arrest and DNA damage-inducible gene 34 (GADD34) or MyD116, PP1c dephosphorylates eIF2α to reduce ATF4 expression [3]. ATF4 plays several critical roles in cellular physiology. For example, ATF4 expression is induced as a component of the integrated stress response (ISR) that regulates cellular amino acid supply, protects against oxidative stress [4], and supports normal postsynaptic function [5,6]. The ISR is implicated in several disease states [7] such as obesity and diabetes [8].
Type 2 diabetes is a risk factor for Alzheimer’s disease (AD) [9] and substantially increases the risk of cognitive decline in individuals with an apolipoprotein E (ApoE) α4 allele [10]. AD is a neurodegenerative disorder that involves the deposition of pathological amyloid β (Aβ) fragments and neurofibrillary tangles in the brain, presumably leading to impaired memory and cognitive function [11]. The pathological Aβ fragment, Aβ1-42, is generated by α-secretase complex cleavage of amyloid precursor protein (APP), which includes presenilins 1 and 2 (PS1 and PS2), nicastrin, Aph-1, and Pen-2 [12]. Previously, we showed that endoplasmic reticulum (ER) stress [13] and autophagy impairment [14] increased Aβ production via α-secretase activation, which was mediated by increased binding of ATF4 to the promoter region of PS1 to increase PS1 gene expression [15]. Moreover, it is reported that memory deficits and Aβ deposition are exacerbated in high fat dietinduced murine models of AD with obesity [16], and in the offspring of AD mice crossed with diabetic ob/ob mice [17,18]. Taken together, these findings suggest that ER stress, and specifically the ISR, may play a role in AD-related memory deficits [19,20]. In support of this hypothesis, ISR inhibitor (ISRIB) has been shown to regulate eIF2β guanine nucleotide exchange factor (GEF), reduce ATF4 expression in vitro, and promote memory function in wild-type mice in vivo [21,22].
Currently, it is unknown whether ATF4 is involved in obesityinduced memory impairment; however, ATF4 expression is increased in the brains of an aged APP23 AD mouse model [23] and AD patients [24]. Moreover, phosphorylated PERK is observed in AD patient neurons [25]. Furthermore, the cognitive impairment that is related to obesity and/or diabetes may be mediated by interleukin (IL) 1α [26] or deficits in glucocorticoid-mediated hippocampal neurogenesis [27]. ATF4 expression is induced by IL1α with IL6 in pancreatic islets [28] and by insulin in the presence of glucocorticoid in mouse L cells [29], therefore increased ATF4 as a consequence of obesity and/or diabetes may be involved in related cognitive impairments.
In the present study, we evaluated memory impairments in early-stage AD model obese mice, and evaluated the ability of dietary quercetin to ameliorate these impairments in an ATF4- dependent manner.
Methods
Animals
APP23 mice, which express a human APP751 cDNA with a Swedish double mutation on a C57BL/6 genetic background [30], were kindly provided by Dr. M. Staufenbiel (Novartis Pharma, Ltd.; Basel, Switzerland). Obese and diabetic db/db (Leprdb/db) [31,32] mice on a C57BL/6 genetic background was purchased from The Jackson Laboratory (Bar Harbor, ME). To generate obese AD model mice (APP; db/db), hemizygous APP23 mice with Leprdb/+ were crossed with Leprdb/+ mice. All mice were individually housed in a temperature- and lightcontrolled room (24°C; 12-hour light/dark cycle) and provided with water and 4 g/day of AIN93G diet (Table 1, Oriental Yeast Co., Ltd.; Tokyo, Japan). AIN93G diet containing 0.5% quercetin was fed at 7 months of age. Blood glucose measurements were performed using the Freestyle Freedom Lite apparatus (Nipro; Osaka, Japan). All animal studies were approved by the Gifu University Graduate School of Medicine Animal Care and Use Committee and conducted according to the Guidelines for Experiments on Animals provided by the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
| Ingredient (g/100 g diet) |
AIN-93G |
| Casein |
20 |
| L-cystine |
3 |
| Vitamin mixture 1 |
1 |
| Mineral mixture 2 |
3.5 |
| Corn Starch |
39.486 |
| α -Corn Starch |
13.2 |
| Soybean oil |
7 |
| Choline bitartrate |
0.25 |
| Sucrose |
10 |
| Cellulose |
5 |
| t-Butyl Hydroquinone |
0.0014 |
1AIN-93 Vitamin mixture (mg/kg diet): Vitamin A-800 (400,000U); Vitamin B12-2.5; Vitamin D3-250 (100,000U); Vitamin E-7,500; Vitamin K-75; Thiamin HCl-600; Riboflavin-600; Pyridoxine-HCl 700; Nicotinic acid-3,000; Calcium pantotenate -1,600; Folic acid-200; D-Biotin 20; Sucrose 974,655
2AIN-93 Mineral Mixture (mg/kg diet): Calcium carbonate 357,000; Potassium phosphate Monobasic-196,000; Potassium citrate monohydrate 70,780; Sodium chloride 74,000; Potassium sulfate 46,600 Magnesium oxide 24,000; Ferric citrate 6,060; Zinc carbonate 1,650; Manganous carbonate 630; Copper (II) Carbonate monohydrate 324; Potassium iodate 10; Sodium selenate 10.25; Ammonium molybdate tetrahydrate 7.95; Sodium metasilicate Nonahydrate 1,450; Chromium (III) Potassium Sulfate dodecahydrate 275; Lithium chloride 17.4; Boric acid 81.5; Sodium floride 63.5; Nickel (II) Carbonate basic tetrahydrate 30.58; Ammonium vanadate 6.6; Sucrose 221,003.2
Table 1: Ingredients of AIN93G (Oriental Yeast Co., Ltd; Tokyo, Japan) diet.
Behavioral procedures
A Freeze-Frame system (Coulbourn Instruments; Whitehall, PA) and Actimetrics Freeze-Frame software (Coulbourn Instruments) were used for contextual and fear conditioning, as described previously [23]. Briefly, mice (n=7, three female and four male mice per group) were habituated for 20 minutes per day for 3 days. The experiments were performed in chamber A, where foot shocks were delivered through the floor, and chamber B, which had a non-shock floor and bright aluminum sidewalls and where no shocks were delivered. Mice were trained in chamber A and chamber B after habituation every day. Each training trial lasted for 420 seconds and paired three auditory cues (2,800 Hz, 85 dB, 30 seconds) with a coterminating electrical foot shock (0.6 mA for 2 seconds) at the 210-second, 270-second, and 330-second time points. One minute after the last training trial, mice were returned to their home cages. Contextual fear conditioning was assayed 2 and 26 hours after training by monitoring freezing behavior in chamber A for three minutes. Auditory fear conditioning was assayed in chamber B 1.5 hours after the contextual fear conditioning test carried out 26 hours after training. The auditory fear conditioning test involved the presentation of a pre-conditioning stimulus (tone -) for 120 seconds followed by the presentation of the paired auditory cue (tone +) for 120 seconds.
Novel object recognition (NOR) testing was performed as previously described by Ennaceur et al. [33] using the SMART v3.0 tracking system (Panlab, Spain) with video (HDC-HS350; Panasonic; Osaka, Japan). Briefly, mice were habituated for 10 minutes in a white plastic chamber (30 × 30 × 16 cm). On the next day, the mice were exposed to two identical objects (11.5-cm tall 5-cm in diameter filled with beads) placed in proximity to walls with different patterns for 5 minutes per day for 2 days. After a retention interval, one of the objects was replaced with a new object (a 6.5 cm-tall glass pyramid with a 49 cm2 base) in the same position (novel), and the animal’s behavior was observed. Exploration of familiar and novel objects was defined as directing the nose within 2 cm of a given object, as previously described. The behavior of the mouse was monitored using video recording and an automated tracking system (SMART v3.0 software). Objects were cleaned with 70% ethanol and 1% acetic acid between trials.
Western blot
Western blot was performed as described previously [23]. Mouse brain tissues (hippocampus, amygdala, cerebral cortex) were frozen by dry ice and homogenized in buffer A (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM ethylene diamine tetra acetic acid, and 1% Nonidet P-40) containing protease and phosphatase inhibitors. After centrifugation at 13,100 x g, supernatants of hippocampus, amygdala and cerebral cortex were used for western blot. The antibodies used for western blotting were as follows: anti-α -tubulin (Sigma-Aldrich Co., LLC), anti-ATF4 (Santa Cruz Biotechnology, Inc.; Santa Cruz, CA), anti-phosphorylated-cyclic adenosine monophosphate response element binding protein (CREB) (Ser133) and anti- CREB (Cell Signaling Technology; Beverly, CA), and horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobulin G (heavy and light chain) antibodies (Southern Biotech; Birmingham, AL). Quercetin was used for diet supplementation (Extrasynthese; Genay, France; and Sigma-Aldrich Co., LLC). All other chemicals were purchased from Wako Pure Chemical Industries, Ltd., Kanto Chemical Co., Inc., (Tokyo, Japan), and Sigma-Aldrich Co., LLC.
Immunohistochemistry
Immunohistochemistry was performed as previously described [34] and analyzed using an inverted microscope (BZ-9000; KEYENCE, Osaka, Japan). Briefly, APP mice (9 months) and AD; db/db mice (8-9 months) were anesthetized using isoflurane inhalation solution (Pfizer, Groton, CT, USA) and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1-M phosphate buffer (PB). Mouse brains were post-fixed for 2 h in the same 4% paraformaldehyde fixative, and then cryoprotected in 15% sucrose overnight in 0.1-M PB. Sections (14-30 α m) were cut and mounted onto slides with anti-Iba1 (1:1,000 dilution, Wako Pure Chemical Industries, Ltd. Osaka, Japan) in PBS containing 10% normal goat serum (Jackson Immuno Research Laboratories, Inc., West Grove, PA) and 0.1% Triton X-100 for 12 h at 4°C. Alexa Fluor 488-conjugated F(ab)2 anti-rabbit IgG (H + L) (Life Technologies Corporation, Carlsbad, CA, USA) was used to visualize primary antibody signal.
Statistical analysis
Statistical significance (p< 0.05) was determined using Student’s t tests or one-way repeated measures analyses of variance followed by Dunnett post hoc tests when necessary. Data are presented as the mean ± standard error of the mean of 3 independent experiments.
Results
NOR memory is impaired in early-stage APP23 mice with obesity and diabetes
To examine the roles of obesity and diabetes in memory impairment in early-stage AD model mice, we crossed hemizygous APP23 mice expressing a human APP751 cDNA with leptin receptor-mutated obese db/db mice.
In APP23 mice, the earliest detectable Aβ deposition occurs at approximately 6 months of age and region-specific neuronal loss occurs at approximately 14–18 months of age [30]. In our study, Aβ deposits were observed in animals that were older than 11 months of age, but not in animals 6-9 months of age. Body weights and blood glucose levels of double-transgenic APP; db/db mice were significantly increased relative to those of APP mice (Figure 1a). Because ATF4 expression is significantly increased in the brains of APP; db/db mice relative to APP mice at 4-6 months of age [23], we examined the effects of ATF4 on memory impairment in early-stage AD model mice 8-11 months of age. We used two well-established memory tasks, namely, fear conditioning and NOR. For the fear conditioning task, we trained mice to associate a 2-second foot shock with the offset of an auditory stimulus (Figure 1b). APP and APP; db/db mice showed significant increases in contextual fear conditioning (Figure 1c) and auditory fear conditioning (Figure 1d) 1 day after training, although the difference in % freezing in APP; db/db mice after the tone presentation was smaller than that in APP mice. Next, we used the NOR task (Figure 1e) to evaluate episodic-like memory [35]. Exploration time for the novel object was significantly increased in the early-stage AD model mice (Figure 1f). In contrast, APP; db/db mice failed to show a novelty preference after the 3-minute interval. This is indicative of a failure to establish short-term memory (STM) (Figure 1f). This result suggests that obesity and diabetes result in the impairment of episodic-like memory in early-stage APP mice.
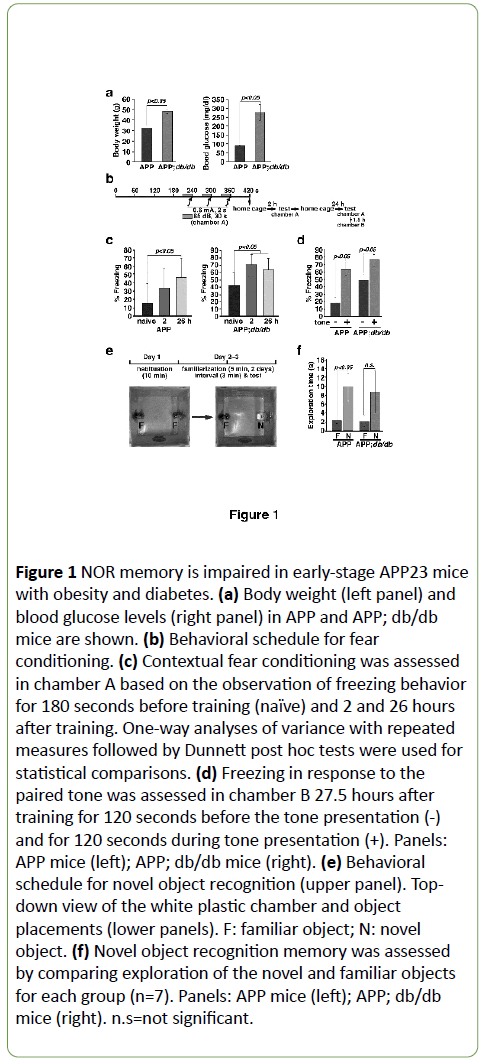
Figure 1: NOR memory is impaired in early-stage APP23 mice with obesity and diabetes. (a) Body weight (left panel) and blood glucose levels (right panel) in APP and APP; db/db mice are shown. (b) Behavioral schedule for fear conditioning. (c) Contextual fear conditioning was assessed in chamber A based on the observation of freezing behavior for 180 seconds before training (naïve) and 2 and 26 hours after training. One-way analyses of variance with repeated measures followed by Dunnett post hoc tests were used for statistical comparisons. (d) Freezing in response to the paired tone was assessed in chamber B 27.5 hours after training for 120 seconds before the tone presentation (-) and for 120 seconds during tone presentation (+). Panels: APP mice (left); APP; db/db mice (right). (e) Behavioral schedule for novel object recognition (upper panel). Topdown view of the white plastic chamber and object placements (lower panels). F: familiar object; N: novel object. (f) Novel object recognition memory was assessed by comparing exploration of the novel and familiar objects for each group (n=7). Panels: APP mice (left); APP; db/db mice (right). n.s=not significant.
Quercetin improves episodic-like memory in early-stage APP23 mice with obesity and diabetes
Recently, Roy and colleagues have shown that memory recall is impaired in a mouse model of early-stage AD, and that engram cell stimulation rescues this impairment [36]. We have also demonstrated that memory recall is improved by dietary quercetin supplementation in a mouse model of AD and in patients with early-stage AD [37]. We thus examined whether obesity and diabetes-related impairments in short-term episodic-like memory in early-stage APP mice are improved by quercetin supplementation. Dietary supplementation with 0.5% quercetin (in AIN93G) for 5 weeks had no effects on body weight (Figure 2a) or blood glucose (Figure 2b) in APP; db/db mice. However, in the NOR task, APP; db/db mice showed an increased preference for the novel object relative to the familiar one (Figures 2c and 2d). Since quercetin has antiinflammatory effects [38] and microglia mediates synapse loss in AD [39], we examined the morphology of microglia by immunostaining using Iba1 antibody according to previously report by Hong et al. [39]. Immuno histochemical analyses indicated that morphology of brain Iba1-positive cells after quercetin supplementation in AD; db/db mice were similarly observed to morphology without quercetin (Figure 2e). Next, we examined the effects of quercetin on ATF4 expression in the brain. ATF4 expression levels in the brain were significantly decreased in APP; db/db mice after quercetin supplementation (Figure 2f) to near wild type levels (data not shown).
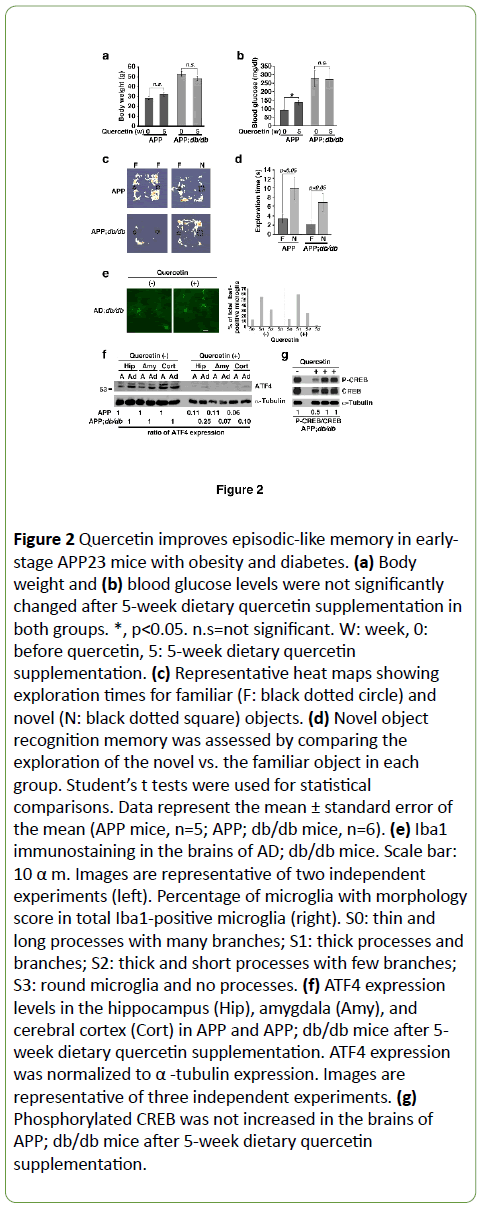
Figure 2: Quercetin improves episodic-like memory in earlystage APP23 mice with obesity and diabetes. (a) Body weight and (b) blood glucose levels were not significantly changed after 5-week dietary quercetin supplementation in both groups. *, p< 0.05. n.s=not significant. W: week, 0: before quercetin, 5: 5-week dietary quercetin supplementation. (c) Representative heat maps showing exploration times for familiar (F: black dotted circle) and novel (N: black dotted square) objects. (d) Novel object recognition memory was assessed by comparing the exploration of the novel vs. the familiar object in each group. Student’s t tests were used for statistical comparisons. Data represent the mean ± standard error of the mean (APP mice, n=5; APP; db/db mice, n=6). (e) Iba1 immunostaining in the brains of AD; db/db mice. Scale bar: 10 α m. Images are representative of two independent experiments (left). Percentage of microglia with morphology score in total Iba1-positive microglia (right). S0: thin and long processes with many branches; S1: thick processes and branches; S2: thick and short processes with few branches; S3: round microglia and no processes. (f) ATF4 expression levels in the hippocampus (Hip), amygdala (Amy), and cerebral cortex (Cort) in APP and APP; db/db mice after 5- week dietary quercetin supplementation. ATF4 expression was normalized to α -tubulin expression. Images are representative of three independent experiments. (g) Phosphorylated CREB was not increased in the brains of APP; db/db mice after 5-week dietary quercetin supplementation.
Since quercetin may enhance phosphorylation of CREB in primary mouse cortical neurons in culture [40] and in mouse brain [41], we measured the levels of phosphorylated CREB in the brains of APP; db/db mice. We found that phosphorylated CREB was not increased after quercetin supplementation (Figure 2g).
Discussion
Although type 2 diabetes is a significant risk factor for Alzheimer’s disease, the underlying mechanisms for this association remain unclear. Here we report that obesity and diabetes enhance ATF4 expression in the brains of early-stage AD model mice and impair short-term episodic-like memory. Moreover, both increased ATF4 expression and STM impairments were improved by dietary quercetin supplementation.
Memory impairment in the NOR task, which is a task that requires no external motivation, reward, or punishment, was exacerbated in APP; db/db mice [35]. Interestingly, NOR memory impairment is ameliorated in 18-week-old db/db mice after treadmill training. This effect is mediated by the inhibition of IL1α -mediated neuroinflammation [26] and is observed in ~11-week-old db/db mice after the normalization of corticosterone levels, which are associated with decreases in fasting glucose levels and insulin concentrations [27]. NOR memory deficits were improved in APP; db/db mice after quercetin supplementation. This effect was correlated with a decrease in ATF4 expression in the brain. Since IL1α and insulin induce ATF4 expression in db/db pancreatic islets [28] and in mouse L cells [29], respectively, quercetin may reduce ATF4 expression by controlling the levels of IL1α and insulin. In Aplysia, micro-RNA (miRNA) 124 and PIWI-interacting RNA-F, which are small noncoding RNAs, regulate serotonin-induced neuronal plasticity through CREB1 translation by reducing the levels of miRNA 124 and suppressing CREB2 (also called ATF4) transcription via the methylation of the promoter for CREB2, respectively [42]. Although we have shown that quercetin suppresses ATF4 translation by reducing eIF2α phosphorylation [23], transcriptional regulation of ATF4 should be assessed in future experiments. The suppression of ATF4 expression may improve synaptic plasticity [43] through CREBdependent transcription [44] pathways, such as those involving brain-derived neurotrophic factor (BDNF) [45]. CREB is involved in the allocation [46] and recall [47] of fear memory, and the activation of subsets of neurons in the amygdala [46,47]. In our experiment, the expression of phosphorylated CREB was not increased in mouse brains after quercetin supplementation, suggesting that quercetin may not affect the expression of phosphorylated CREB.
Conclusion
Several studies have suggested that the ISR is important for the regulation of synaptic plasticity and memory formation [48]. Reduced eIF2α phosphorylation improves memory and neurodegeneration in mice; eIF2α phosphorylation and neuronal loss are increased in mice with prion disease in a manner that is prevented by growth arrest and DNA damagedinducible gene (GADD) [34] overexpression [49]. In addition, NOR impairments related to prion-infected mice are prevented via reduced eIF2α phosphorylation and ATF4 expression by oral PERK inhibitor treatment after inoculation [50]. Additionally, ISRIB, which stimulates eIF2β and reduces ATF4 expression [22], improves memory in 10-week-old wild-type mice [21] and prevents neurodegeneration in mice with prion disease [51]. Consistent with the above findings, we have shown that quercetin induces GADD34 expression and decreases ATF4 expression in the brain [23]. Taken together, these findings and our results suggest that ATF4 may play a role in memory impairment in AD with obesity and diabetes. Therefore, control of the ISR with biologically active compounds that reduce ATF4, such as quercetin, may be valuable for the treatment of memory impairments in earlystage AD, and particularly in cases with comorbid obesity and/or diabetes.
Acknowledgments
The authors declare no financial conflicts of interest with regard to this study or the preparation of the manuscript. We are grateful to Dr. M. Staufenbiel (Novartis Pharma, Ltd.; Basel, Switzerland) for providing APP23 mice and Mrs. M. Hayakawa- Ogura (Gifu University) for technical assistance. This work was supported in part by the Mitsui Life Social Welfare Foundation and Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan (T.N.).
Competing Interests
The authors declare no competing interests with regard to this study or the preparation of the manuscript.
21136
References
- Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101: 11269-1174.
- Holcik M, Sonenberg N (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318-327.
- Novoa I (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. The Journal of cell biology 153: 1011-1022.
- Harding HP (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619-633.
- Pasini S (2015) Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep 11: 183-191.
- Hu JY (2015) cJun and CREB2 in the postsynaptic neuron contribute to persistent long-term facilitation at a behaviorally relevant synapse. J Neurosci 35: 386-395.
- Wang S, Kaufman RJ (2012) The impact of the unfolded protein response on human disease. J Cell Biol 197: 857-867.
- Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900-917.
- Luchsinger JA, Gustafson DR (2009) Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis 16: 693-704.
- Haan MN (1999) The role of APOE epsilon4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA 282: 40-46.
- Mattson MP (2004) Pathways towards and away from Alzheimer's disease. Nature 430: 631-639.
- Selkoe DJ, Wolfe MS (2007) Presenilin: Running with scissors in the membrane. Cell 131: 215-221.
- Ohta K (2011) Endoplasmic reticulum stress enhances gamma-secretase activity. Biochemical and biophysical research communications 416: 362-366.
- Ohta K (2010) Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy 6: 345-352.
- Mitsuda T (2007) ATF4 regulates gamma-secretase activity during amino acid imbalance. Biochemical and biophysical research communications 352: 722-727.
- Maesako M (2012) Exercise is more effective than diet control in preventing high fat diet-induced beta-amyloid deposition and memory deficit in amyloid precursor protein transgenic mice. J Biol Chem 287: 23024-23033.
- Takeda S (2010) Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA 107: 7036-7041.
- Niedowicz DM (2014) Obesity and diabetes cause cognitive dysfunction in the absence of accelerated beta-amyloid deposition in a novel murine model of mixed or vascular dementia. Acta Neuropathol Commun 2: 64.
- Lourenco MV (2015) Neuronal stress signaling and eIF2α phosphorylation as molecular links between Alzheimer's disease and diabetes. Prog Neurobiol 129: 37-57.
- Endres K, Reinhardt S (2013) ER-stress in Alzheimer's disease: turning the scale? Am J Neurodegener Dis 2: 247-265.
- Sidrauski C (2013) Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2: e00498.
- Sekine Y (2015) Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 348: 1027-1030.
- Hayakawa M (2015) Quercetin reduces eIF2α phosphorylation by GADD34 induction. Neurobiol Aging 36: 2509-2518.
- Baleriola J (2014) Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell 158: 1159-1172.
- Hoozemans JJ (2009) The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am J Pathol 174: 1241-1251.
- Erion JR (2014) Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci 34: 2618-2631.
- Stranahan AM (2008) Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci 11: 309-317.
- O'Neill CM (2013) Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology 154: 3077-3088.
- Adams CM (2007) Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 282: 16744-16753.
- Calhoun ME (1998) Neuron loss in APP transgenic mice. Nature 395: 755-756.
- Chen H (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491-495.
- Lee GH (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature 379: 632-635.
- Ennaceur A (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113: 509-519.
- Li S (2012) Olfaxin as a novel Prune2 isoform predominantly expressed in olfactory system. Brain Res 1488: 1-13.
- Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93-110.
- Roy DS (2016) Memory retrieval by activating engram cells in mouse models of early Alzheimer's disease. Nature 531: 508-512.
- Nakagawa T (2016) Improvement of memory recall by quercetin in rodent contextual fear conditioning and human early-stage Alzheimer's disease patients. Neuroreport 27: 671-676.
- Chen S (2016) Therapeutic effects of quercetin on inflammation, obesity, and Type 2 diabetes. Mediators Inflamm 2016: 9340637.
- Hong S (2016) Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352: 712-716.
- Nichols M (2015) Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 308: 75-94.
- Tchantchou F (2009) Stimulation of neurogenesis and synaptogenesis by bilobalide and quercetin via common final pathway in hippocampal neurons. J Alzheimers Dis 18: 787-798.
- Alberini CM, Kandel ER (2014) The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol 7: a021741.
- Costa-Mattioli M (2007) eIF2-alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129: 195-206.
- Chen A (2003) Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron 39: 655-669.
- Lu Y (2008) BDNF: A key regulator for protein synthesis-dependent LTP and long-term memory? Neurobiol Learn Mem 89: 312-323.
- Zhou Y (2009) CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci 12: 1438-1443.
- Kim J (2014) Memory recall and modifications by activating neurons with elevated CREB. Nat Neurosci 17: 65-72.
- Costa-Mattioli M (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61: 10-26.
- Moreno JA (2012) Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature 485: 507-511.
- Moreno JA (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med 5: 206ra138.
- Halliday M (2015) Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 6: e1672.







