Keywords
Digital medicine system; Aripiprazole (Abilify Mycite); Adherence
Introduction
The Digital Medicine System (DMS) is an innovative drug–device combination developed for patients with serious mental illness. This integrates adherence measurement capabilities as part of the drug formulation via an embedded ingestible sensor [1]. Serious Mental Illness (SMI) is one of the leading causes of longterm disability worldwide. Major Depressive Disorder (MDD), schizophrenia, and bipolar disorder were ranked second, 11th, and 17th, respectively, on the list of global causes of long-term disability surveyed in 188 countries by the Global Burden of Disease Study 2013. SMI affects a large proportion of the US population; in 2012, the estimated prevalence of mental illness and SMI among adults was 18.5% and 4.2%, respectively, based on the National Survey on Drug Use and Health. Pharmacologic treatment interventions reduce the severity of SMI and improve patient outcomes [2]. Numerous modalities currently available for direct or objective assessment of adherence, including pill counts, Medication Event Monitoring System (MEMS) bottle caps, pharmacy refill records, and biological assays from bodily fluids have limitations, and none provide an actual measure of medication ingestion. Therefore, the ability to precisely and objectively assess medication adherence in patients with SMI remains a significant unmet need. A newly developed Digital Medicine System (DMS) offers an innovative opportunity to objectively measure and report actual patient medication adherence [3]. Emerging digital technologies are one of the fastest growing sectors within the healthcare industry and are starting to reshape our perspective on how some diseases can be managed [4]. Analytical electrochemistry is finding a broad range of applications in the biomedical field. For example, there have been developments in different bio and electrochemical sensors such as: nitric oxide sensors, glucose sensors, DNA sensors, hydrogen sulphide sensors, oxygen sensors, superoxide sensors, immune sensors etc. [5]. A new field of digital medicine slackly defined as the field of medicine that uses digital tools to promote the practice of medicine to one that is high-definition and far more individualized has emerged [6].
Literature Review
Following the landmark regulatory approval of digital aripiprazole (Abilify Mycite™) latein 2017, digital medicines have begun the transition from clinical trials and early clinical success to mainstream utilization. The ingestible sensor enabling the era of Digital Medicines was formerly approved in 2012 via FDA’s de novo pathway for novel low-risk devices; commercial use began shortly thereafter [7,8].
The technology of digital medicine system
Digital medicines aim to improve pharmaceutical therapy by improving patient adherence [8]. The use of Smartphone based data streams in relation to mental health research is steadily gaining traction in the field [9]. The latest technology is the use of a digital medicine system in which a drug is combined with an ingestible sensor that can transmit a signal when the drug-device combination is exposed to gastric acid in the stomach to allow factual- time information about medication ingestion. It is hoped that this will increase medication adherence and in turn effect in improved health outcomes and be cost-saving [10]. Digital medicine systems have the potential to provide several benefits to patient care, including increasing medication adherence, providing real- time tracking of drug use and extenuating risks of overdose and abuse [11,12]. The DSM is a personal digital tool (Smartphone) which wirelessly connects to the patient’s documentation and mechanically uploads their medication prescriptions [11]. Wireless device with electrochemical sensing techniques can be used alternatively [5]. The digital medicine consists of an ingestible sensor of 1 (mm)2 in size that is placed in an oral solid preparation such as a tablet, a wearable sensor patch, and a mobile computing device. The sensor is coated with digestible metals such as copper and magnesium. Once ingested and activated by gastric fluid, the sensor generates an indication that is perceived by the patch. The amount of copper and magnesium that can be absorbed by the intestine from the ingestible sensor is very small compared with their daily allowable amounts for human consumption of 0.3% (7.7 mg) and 0.003% (9.8 mg), respectively. The wearable sensor patch is a body-worn sensor of approximately 10 cm in length that detects and records the date and time of medication ingestion. A foam surface holds the adhesive and provides a water-resistant enclosure for the device’s electronics. It should be applied on the upper body (torso), and it can be worn throughout most activities, including exercise and bathing. High levels of activity and water may cause the patch to strip off, so the adhesive should be replaced weekly [13]. A novel Digital Medicine System (DMS) has been developed for patients with serious mental illness to objectively measure and report ingestion of aripiprazole, an atypical antipsychotic [3]. DMS acts as smart medicine reminder; smart medicine reminder system is designed for, but not restricted to, helping old people in pleasing them- selves in taking their medications at the exact time and in the correct amount [14]. System takes up the prescription details from the user such as the duration of the prescription, the names of the medicines, the period they are to be taken and the amount of each medicine which is to be taken. After all this data has been entered, system will remind the user at the prescribed time of which medicine is to be taken in form of a mobile notification and a physical reminder [14]. Process of digital medicine system is given in Figure 1.
Figure 1: Process of digital medicine system.
Digital pills: Smart reminders
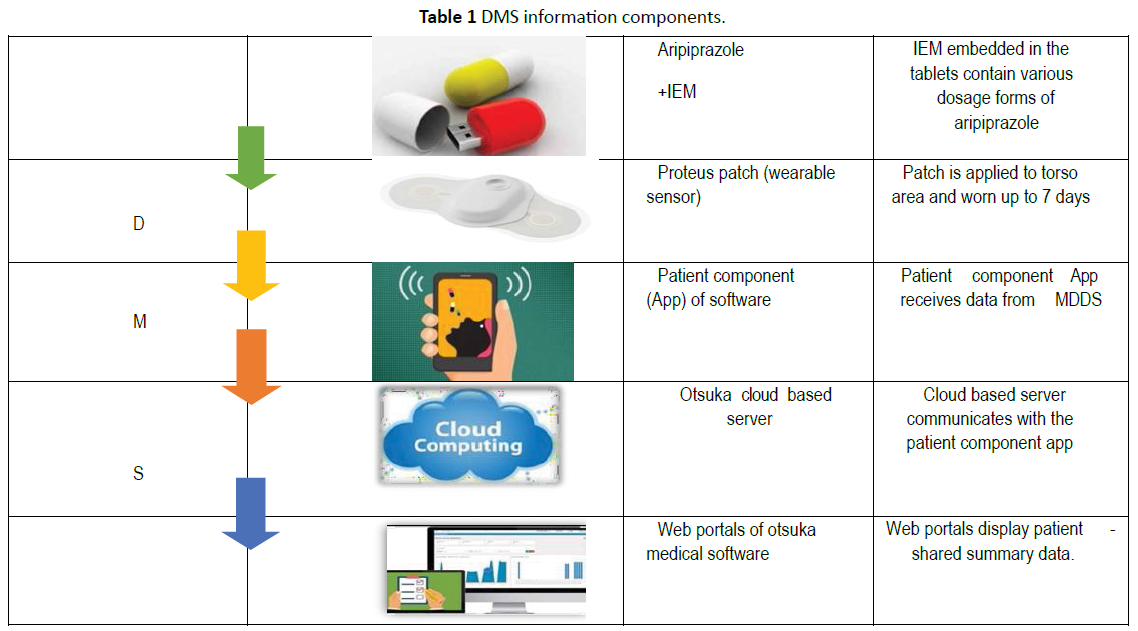
Digital pills consist of a gelatin capsule containing a digital radiofrequency emitter compounded with the desired medication. When ingested, the radiofrequency emitter is activated by the chloride ion gradient in the stomach to transmit a unique signal. A hip-worn receiver detects this signal before relaying data regarding the identity of the ingested medication and time of ingestion through third-generation (3G) cellular signalling to a cloud-based server compliant with Health Information Technology for Economic and Clinical Health (HITECH) and the Health Insurance Portability and Accountability Act (HIPAA). Digital pills are keyed up by the specific chloride ion gradient in the stomach and, therefore, cannot be activated outside of the body. Because each digital pill emits a unique frequency, the system can record multiple simultaneous ingestion events. In the USA, the Food and Drug Administration evaluates both drugs and medical devices [15]. Newly Food and Drug Administration (FDA) approved drug- device combinations include a smart insulin pen, a smart pill, and a smart inhaler [16]. Digital pills are innovative and simple to operate and passively measure adherence, decreasing the need for an individual to interact with technology to transmit adherence data [17]. Although the DSM technology has been mostly tested in individuals with physical diseases the first digital medicine released on the market is an antipsychotic. On November 13, 2017 the Food and Drug Administration (FDA) approved ‘Abilify MyCite’ as the first drug [18]. With a digital ingestion tracking system [19-21]. This antipsychotic medication is equipped with an ingestible sensor which communicates with a wearable patch, a Smartphone app, and an online portal. Abilify MyCite as an assemblage of different technologies: trackers, sensors, patches, apps, programming, Smartphone, and the internet. It draws on science and technology studies (STS) and new materialism to suggest that in the era of digital mental health, ingestible sensors and other new pharmaceuticals are producing a data-driven subjectivity-a term that refers to the inextricability of data, its processing and production, and the formation of subjectivity-which has the potential to transform mental health care [22]. Aripiprazole is an atypical antipsychotic agent with an intrinsic dopamine agonist activity of 30%. Aripiprazole exerts supplementary partial agonist action on 5-HT1A receptors and has antagonist properties at 5-HT2A receptors [23]. The ingestible sensors are detected with ~98% sensitivity and with 100% correct identification as reported on the current Proteus Discover® Label [8]. Proteus Digital Health, Inc, is a company that develops and commercializes the ingestible sensor and wearable sensor patch that obtained FDA approval in 2012. Through their joint venture with Otsuka Pharmaceutical, they developed the Abilify® (aripiprazole) digital medicine [24]. This is the first time a new drug application for a digital medicine is accepted for revision by the FDA. When the Abilify® (aripiprazole) digital medicine is taken, the sensor sends a signal to the wearable sensor patch after it reaches the stomach [25]. This information is recorded and relayed to patients on a mobile phone or other Bluetooth- enabled device and only with their consent to their physicians and/or their caregivers [13]. Abilify MyCite, is used in the treatment of schizophrenia, bipolar disorder, and depression [26]. It combines aripiprazole with a digital sensor that, once in the stomach, communicates with a patch, worn by the patient that automatically logs the date, time, and dosage of the medication [26]. Different methods of DMS components are given in Table 1.
Cautions while using medication
1. Do not set up a new patch until instructed by the app.
2. Do not take first DMS pill until instructed by the app.
3. Do not stop or change medication dosage based on information provided by the DMs kit; consult health care provider.
4. Do not alter medication unless instructed by prescribing physician Keep DMS components out of the reach of children.
5. Do not chew pill.
6. Do not place pill in water.
7. Do not use the patch if allergic to adhesive [1].
Information transmission steps are given in Figure 2.
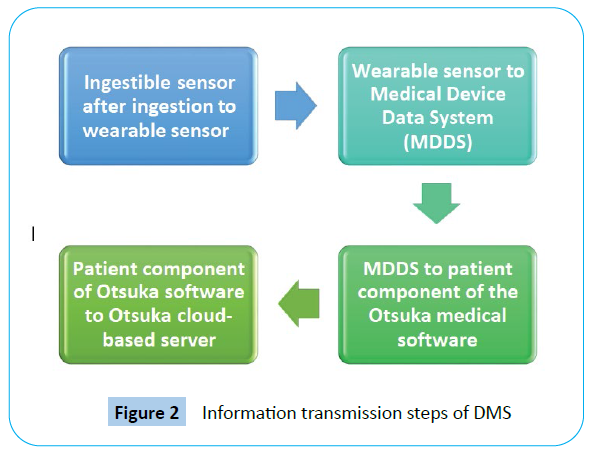
Figure 2: Information transmission steps of DMS
Digital health revolution: perfect opportunities for pharmaceutical R & D
Utilizing big data, sensors and artificial intelligence and software platforms provides the chance for improved disease management across multiple disease areas. Pharmaceutical companies are aware and are looking at ways in which these types of technologies can complement their products to offer “beyond the pill” solutions. Some Health Technology Assessment (HTA) bodies in Europe have started to look at how they can evaluate digital technology solutions alone or in combination with pharmaceutical products; however, access pathways remain unclear posing a hindrance for manufactures trying to deliver solutions and benefits to patients [4]. The increasingly wide array of available digital technologies presents pharma with multiple opportunities throughout its business operations. For R & D, it offers potential to not only generate new therapeutic products and services but also to improve the drug development process itself. Compared with market-oriented activities, pharma’s adoption of digital technologies to enhance clinical trials and drug efficacy are still at the early stages [27].
Medication adherence
Adherence is a contested concept insofar as it is imbued with meanings that extend beyond its strict [26,27]. Adherence is the degree to which a patient follows medical advice, most commonly with regard to taking medications [28]. Monitoring of pill consumption compliance and adherence may be optimized [29]. Digital medicine sensors improve the medication adherence [30,31]. Products that incorporate adherence monitoring are already on the market and others are awaiting FDA approval. For example, Rush University is using a compound pharmacy to combine existing medications with an ingestible sensor, developed by Proteus Digital Health, Otsuka Pharmaceuticals’ recent FDA approval of a combination of the Proteus sensor with the drug Abilify (i.e., aripiprazole, which is currently FDA approved for a range of indications in the treatment of serious mental illnesses) [28]. Current adherence modalities are explained in Figure 3.
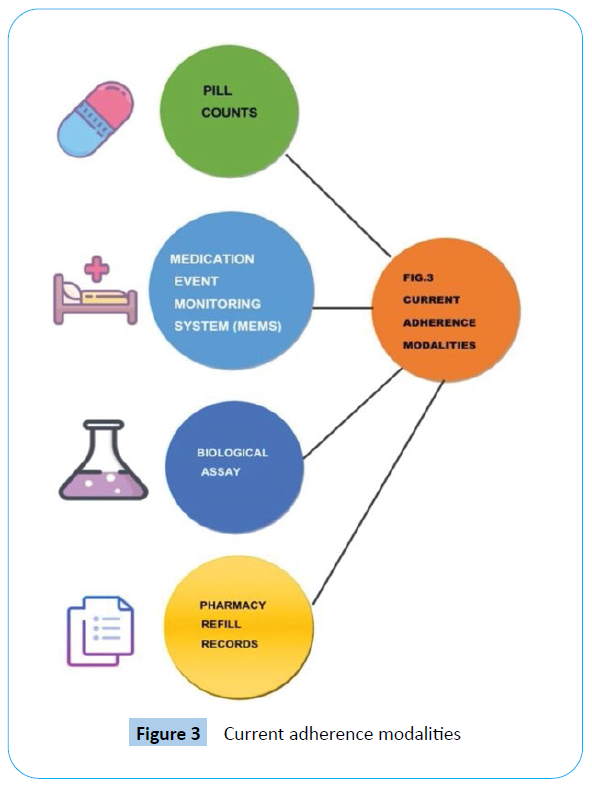
Figure 3: Current adherence modalities
Limitations
Nonadherence to medication is a common concern: Incorrect medication intake can lead to suboptimal health for the patient and, counter- intuitively, may increase health-related costs [32].
Privacy: Patients have to grant consent in order for the information to be shared with health care providers.
Cost: This medication is likely cost significantly more than the generic version, making it inaccessible for many patients or limited to clinical trials [33]. Poor medication adherence produces large down- stream health care costs [34].
Discussion
Digital medicines can represent a future for cardiovascular healthcare helping to identify barriers in the implementation of preventative and therapeutic interventions [35,36]. Miniaturization and wirelessization are the key Product development innovations [37]. The development of smaller and more accurate sensors, integrated with the computing power, data storage, and transfer capabilities of current personal handheld communication devices, holds the promise of very powerful diagnostic and monitoring tools. Development of technology and devices in health care encounters the obvious challenges of research and development.
Conclusion
DMS sets the basis for future research concerning both the potential and concerns related to a wider use of digital pill and digitalisation of traditional drugs. DMS represents the first integrative digital health product developed in psychiatry, in alignment with FDA HF guidance. It provides substantial benefits not achievable with common clinical approaches to address adherence issues. Digital Medicine System could help transform unsustainable healthcare systems into sustainable ones. They will surely signal the new age of healthcare in future.
Acknowledgements
Authors are thankful to management and Principal, GES’s Sir Dr. M.S. Gosavi College of Pharmaceutical Education and Research, Nashik-422005, Maharashtra, India.
38459
References
- Peters-Strickland T, Hatch A, Adenwala A, Atkinson K, Bartfeld B (2018) Human factors evaluation of a novel digital medicine system in psychiatry. Neuropsych Dis Treat 14: 553.
- Martani A, Geneviève LD, Poppe C, Casonato C, Wangmo T (2020) Digital pills: a scoping review of the empirical literature and analysis of the ethical aspects. BMC med ethics 21: 1-3.
- Peters-Strickland T, Pestreich L, Hatch A, Rohatagi S, Baker RA, et al. (2016) Usability of a novel digital medicine system in adults with schizophrenia treated with sensor-embedded tablets of aripiprazole. Neuropsych Dis Treat 12: 2587.
- Focsa S (2017) What can be done for access and reimbursement processes to reward innovation in digital “beyond the pill” solutions?. Value Health 20: A708.
- Mc Caffrey C, Twomey K, Ogurtsov VI (2015) Development of a wireless swallowable capsule with potentiostatic electrochemical sensor for gastrointestinal track investigation. Sensor Actuat B-Chem 218: 8-15.
- Venuturupalli RS, Sufka P, Bhana S (2019) Digital medicine in rheumatology: challenges and opportunities. Rheum Dis Clin 45: 113-26.
- Vayena E, Ienca M (2018) Digital medicine and ethics: rooting for evidence. Am J Bioeth 18: 49-51.
- Plowman RS, Peters-Strickland T, Savage GM (2018) Digital medicines: clinical review on the safety of tablets with sensors. Expert Opin Drug Saf 17: 849-852.
- Mulder T, Jagesar RR, Klingenberg AM, Bonnici JP, Kas MJ (2018) New European privacy regulation: Assessing the impact for digital medicine innovations. Eur Psych 54: 57-58.
- Cosgrove L, Cristea IA, Shaughnessy AF, Mintzes B, Naudet F (2019) Digital aripiprazole or digital evergreening? A systematic review of the evidence and its dissemination in the scientific literature and in the media. BMJ Evid Based Med 24: 231-238.
- Akkermans J, de Lange AH, van der Heijden BIJM, Kooij DTAM, Jansen PGW, Dikkers JSE (2016) "What about time? Examining chronological and subjective age and their relation to work motivation". Career Dev Int 21: 419-439.
- Egilman AC, Ross JS (2019) Digital medicine systems: an evergreening strategy or an advance in medication management?. BMJ Evid Based Med 24: 203- 204.
- Vallejos X, Wu C (2017) Digital medicine: innovative drug-device combination as new measure of medication adherence. J Pharm Tech 33: 137-139.
- Abdul Minaam DS, Abd-El-Fattah M (2018) Smart drugs: Improving healthcare using Smart Pill Box for Medicine Reminder and Monitoring System. Future computing inform J 3: 28.
- Frigerio M (2016) Getting approval for new therapeutic medical devices versus drugs: are the differences justified?. Eur Resp Rev 25: 223-226.
- Zijp TR, Mol PG, Touw DJ, van Boven JF (2019) Smart Medication Adherence Monitoring in Clinical Drug Trials: A Prerequisite for Personalised Medicine?. EClinical Medicine 15: 3-4.
- Chai PR, Carreiro S, Innes BJ, Rosen RK, O'Cleirigh C, et al. (2017) Digital pills to measure opioid ingestion patterns in emergency department patients with acute fracture pain: a pilot study. J Med Internet Res 19: e19.
- Dotolo D, Petros R, Berridge C (2018) A hard pill to swallow: ethical problems of digital medication. Social work 63: 370-372.
- Papola D, Gastaldon C, Ostuzzi G (2018) Can a digital medicine system improve adherence to antipsychotic treatment?. Epidemiol Psych Sci 27: 227-229.
- Hunt S, Hellwig JP (2018) FDA Approves First Digital Medicine System. Nurs Women's Health 22: 12.
- Voelker R (2018) News from the food and drug administration. J Am Med Asso 320: 23.
- Flore J (2020) Ingestible sensors, data, and pharmaceuticals: Subjectivity in the era of digital mental health. New Media Soc. 2020: 1461444820931024.
- Fischer B, Davids E, Gastpar M (2004) Aripiprazol-Pharmakologie eines neuen atypischen Antipsychotikums. Fortschr Neurol Psychiatr 72: 497-502.
- Van Biesen W, Decruyenaere J, Sideri K, Cockbain J, Sterckx S (2021) Remote digital monitoring of medication intake: methodological, medical, ethical and legal reflections. Acta Clin Belg 76: 209-216.
- Daar ES, Rosen MI, Wang Y, Siqueiros L, Shen J, et al. (2020) Real‐time and wireless assessment of adherence to antiretroviral therapy with co‐encapsulated ingestion sensor in HIV‐infected patients: A pilot study. Clin transl sci 13: 189-94.
- Swartz AK (2018) Smart pills for psychosis: the tricky ethical challenges of digital medicine for serious mental illness. Am J Bioethics 18: 65-67.
- Hird N, Ghosh S, Kitano H (2016) Digital health revolution: perfect storm or perfect opportunity for pharmaceutical R&D?. Drug Discov Today 21: 900-911.
- Klugman CM, Dunn LB, Schwartz J, Cohen IG (2018) The ethics of smart pills and self-acting devices: Autonomy, truth-telling, and trust at the dawn of digital medicine. Am J Bioethics. 18: 38-47.
- Young MA, Dimartino L (2018) Trackable pill digital technology in PRM & pain: Hype or hope?. Ann Phys Rehabil Med 61: e115.
- Vickers NJ (2017) Animal communication: when i’m calling you, will you answer too?. Current Biol 27: 713-715.
- Batra S, Baker RA, Wang T, Forma F, Di Biasi F, et al. (2017) Digital health technology for use in patients with serious mental illness: a systematic review of the literature. Med. Devices: Evid Res 10: 237.
- Kopelowicz A, Baker RA, Zhao C, Brewer C, Lawson E, Peters-Strickland T (2017) A multicenter, open-label, pilot study evaluating the functionality of an integrated call center for a digital medicine system to optimize monitoring of adherence to oral aripiprazole in adult patients with serious mental illness. Neuropsych Dis Treatment 13: 2641.
- Jongsma KR, Bredenoord AL, Lucivero F (2018) Digital medicine: an opportunity to revisit the role of bioethicists. Am J Bioeth 18: 69-70.
- Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ (2012) Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Annals Int Med. 157: 785-95.
- Banchs JE, Scher DL (2015) Emerging role of digital technology and remote monitoring in the care of cardiac patients. Med Clinics 99: 877-96.
- El-Hadidi S, Rosano G (2020) Evidence beyond the digital medication pill’, Eur Heart J- Cardiovasc Pharmacother 6: 72-74.
- Onodera R, Sengoku S (2018) Innovation process of mHealth: An overview of FDA-approved mobile medical applications. Int J Med Inform 1: 65-71.










