Keywords
flow cytometry, stem cells, CD34+ antigen, cord blood, white blood cells, viability
Introduction
Flow cytometer system analyze biological specimen in order to identify various cell populations determined by the specific monoclonal antibodies and fluorochromes used.
A flow cytometer is made up of three main systems: fluidics, transports particles in a stream to the laser beam for interrogation, optics, consists of lasers to illuminate the particles in the sample stream and optical filters to direct the resulting light signals to the appropriate detectors, and electronics, converts the detected light signals into electronic signals that can be processed by the computer [1-3] .
In the flow cytometer, particles are carried to the laser intercept in a fluid stream. Any suspended particle of cell from 0.2-150 micrometers in size is suitable for analysis.
The characteristics or parameters of each event are based on its light scattering and fluorescent properties [3,4]. The data are collected and stored in the computer. This data can be analyzed to provide further information about subpopulations within the sample.
Stem cells have the remarkable potential to develop into many different cell types (self differentiation) in the body. Serving as a sort of repair system for the body, they can theoretically divide without limit (self renewal) to replenish other cells as long as the person is still alive [5-8]. Adult stem cells are currently the only type of stem cells commonly used to treat human diseases [9-11]
When a stem cell divides, each new cell has the potential to either remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, or a red blood cell [12-15] The CD34+ antigen is a family of differentially glycosylated type I transmembrane single hain glycoproteins (see diagram 1). It is expressed on virtually all haematopoietic precursor cells. Including multipotent CD34+ epitopes have been separated into three classes based upon sensitivity to neuraminidase and to pasteurella heamolytica glycoprotease [16-18] .
Diagram 1: CD34+ Physical map.
A single platform flow cytometry method is available for rapid determination of absolute CD34+ cells, based on guidelines developed by the International Society of Hematotherapy and Graft Engineering (ISHAGE) [19,20] .
Viability of mononuclear cells is determined using the exclusion dye 7-AAD which emits in the far red spectrum and can be easily combined with absolute CD34+ cell counting to enumerate viable CD34+ cells.
The data interpretation in this study for CD34+ cell enumeration and viability testing shows high accuracy, providing important validation of the single platform methodology.
Method
Prepared single cell or particle suspensions are necessary for flow cytometric analysis. Various immunoflurescent dyes or antibodies conjugates can be attached to the antigen of interest; in our study 7-AAD was used.
The suspension of cells is aspirated into a flow cell where, surrounded by a narrow sheath fluid, they pass one at a time through a focused laser beam.
The light is either scattered or absorbed when strikes a cell. Absorbed light of the appropriate wavelength may be re-emitted as fluorescence if the cell contains a naturally fluorescent substance of one or more fluorochrome-labelled antibodies are attached to surface or internal cell structure. Light scatter is dependent on the internal structure of the cell and its size and shape.
Light scattered in the forward direction is collected by the Forward Scatter (FS) photodiode and provides information on the relative size of the particle (see diagram 2). Light measured at a 90° angle to the laser excitation beam is collected by the Side Scatter detector (SS) provides information on the relative granularity of the particle. PMT's separate fluorescence (FL-) detects any fluorescent light associated with the particle. The light is directed via a series of mirrors and filters to PMT´s. Filters determine the wavelength of light to be detected by the PMTs, any unfiltered light is reflected by a dichoric filter and is channelled to the next fluorescent channel.
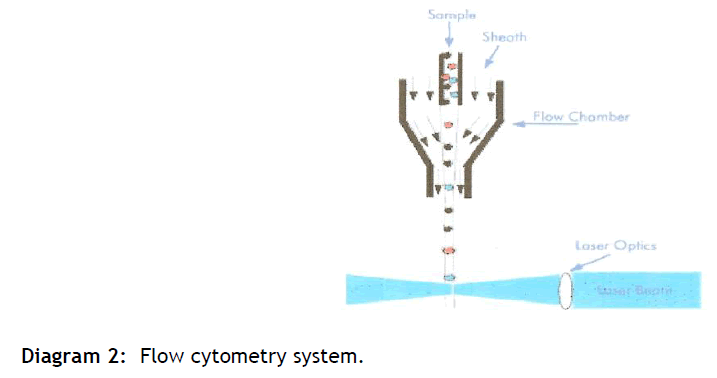
Diagram 2: Flow cytometry system.
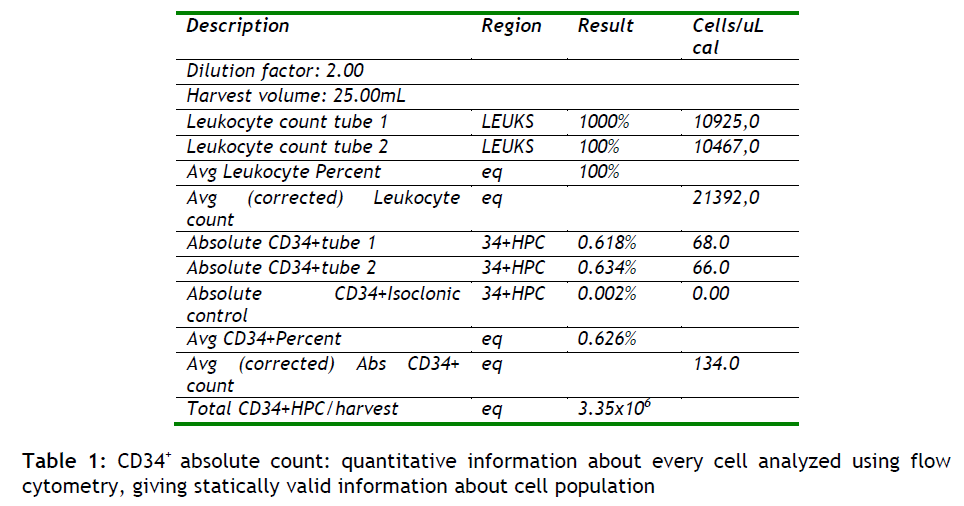
The resulting electrical pulses are digitized, and he data is stored, analyzed, and displayed through a computer system. The end result is quantitative information about every cell analyzed. Since large numbers of cells are analyzed in a short period of time (>1,000/sec), statistically valid information about cell population is quickly obtained (see table 1).
Assessing CD34+ cell viability and absolute counts, at different stages of processing allows for accurate quality control. Alterations in the cryopreservation or collection technique can be spotted in their effect on CD34+ cell viability and absolute counts. Also, products that are cryopreserved for lengthy periods, such as cord blood samples, can be assessed for quality using the single platform flow cytometry methodology.
Flow cytometric analysis was performed on post freeze cord blood samples, using Stem-kitTM (Beckman Coulter FC 500), for the simultaneous identification and enumeration of CD45+ and dual-positive CD45+CD34+ cell population percentages and absolute counts in cord blood. Samples were analyzed sing the double-colour single platform variant of the ISHAGE guidelines with the addition of the viability dye 7-AAD. This method allows as the measurement of viable CD34+ cells directly by the flow cytometer without the need for a haematology analyzer, and a direct assessment of total WBC and CD34+ cell viability in a single tube. In addition, specimens (n = 4000) were also analyzed on an automated haematology analyzer for the determination of total WBC, (required for the calculation of absolute numbers of CD34+ cells), and then the results were compared with the WBC obtained from the flow cytometry analyzer (see Table 5).
Stem-kitTM (Beckman Coulter-Immunoteck) which utilizes CD45-FITC/CD34-PE and Flow-CountTM Fluorospheres was used for determining absolute CD34+ counts. This test depends on the ability of a monoclonal antibody (mAb) to bind to the surface of cells expressing iscrete antigenic determinants. Also we used an FITC- conjugated mAb to the CD45 antigen which not only will detect all isoforms but also all the glycoforms. In a combination now, with the PE-conjugated antiCD34, the anti-CD45FITC mAb will provide an additional parameter for the identification of CD34+ cells. This mAb combination enables the identification of true CD34+ cells. Prior to sample preparation we ensure that the white blood cells (WBC) concentration was not greater than 30x109 WBC cells/L.
Briefly, umbilical cord blood samples (n = 4000) were collected (collection volume > 120ml) into a modified blood collection pack and processed within 36h of collection. Cord blood was centrifuged to obtain the leukocyte-rich buffy coat layer. An aliquot of the cellular fraction was used for CD34+ cell absolute counts, WBC, and viability. All procedures used for collecting and processing cord blood samples were based on the standard operating procedures of the American Cryobanks International with accreditation from the AABB (American Association of Blood Banks) and from the FDA (Food and Drug Administration).
To assess thawed cord blood countenance in CD34+ cells absolute numbers and their viability, in every step of processing, three-colour flow cytometry was used. In our study we used multi-parameter flow cytometry to determine the absolute number and viability of 4000 cryopreserved cord blood samples stored in liquid phase nitrogen of -192°C. We divide the samples, prior to counting CD34+ cells absolute numbers and their viability, as follow: 1) 2500 cryopreserved cord blood samples stored in liquid phase nitrogen of -192°C for one year, 2) 1000 cryopreserved cord blood samples stored in liquid phase nitrogen of -192°C for 6 months, 3) 250 cryopreserved cord blood samples stored in liquid phase nitrogen of -192°C for 3 months and 4) 250 cryopreserved cord blood samples stored in liquid phase nitrogen of -192°C for 24 hours.
Procedure for specimen processing:
One hundred μl thawed cord blood sample was added to 20μl of CD45-FITC/CD34-PE and 20μl of 7-AAD (1μg/ml final concentration). All samples were prepared in duplicate(45/34/7-AAD # 1 and 45/34/7-AAD # 2) and one control tube (45/CTRL/7-AAD) containing 100μl of thawed cord blood sample and 20μl of CD45-FITC/IsoClonicTM Control-PE reagent.
Tubes were then vortex for 5 seconds and incubated for 20min in a dark place, protected from light, at room temperature (18-25°C). Immediately before analysis 20μl of phosphate buffered saline (PBS) and a 100μl of Flow-CountTM Fluorospheres were added, all tubes vortex for 5 seconds, and analyzed on a Beckman Coulter FC-500 flow cytometer. Sample analysis was completed typically within 30 min.
A total of 75.000 CD45+ events were collected per tube (see figure 1, table 1).
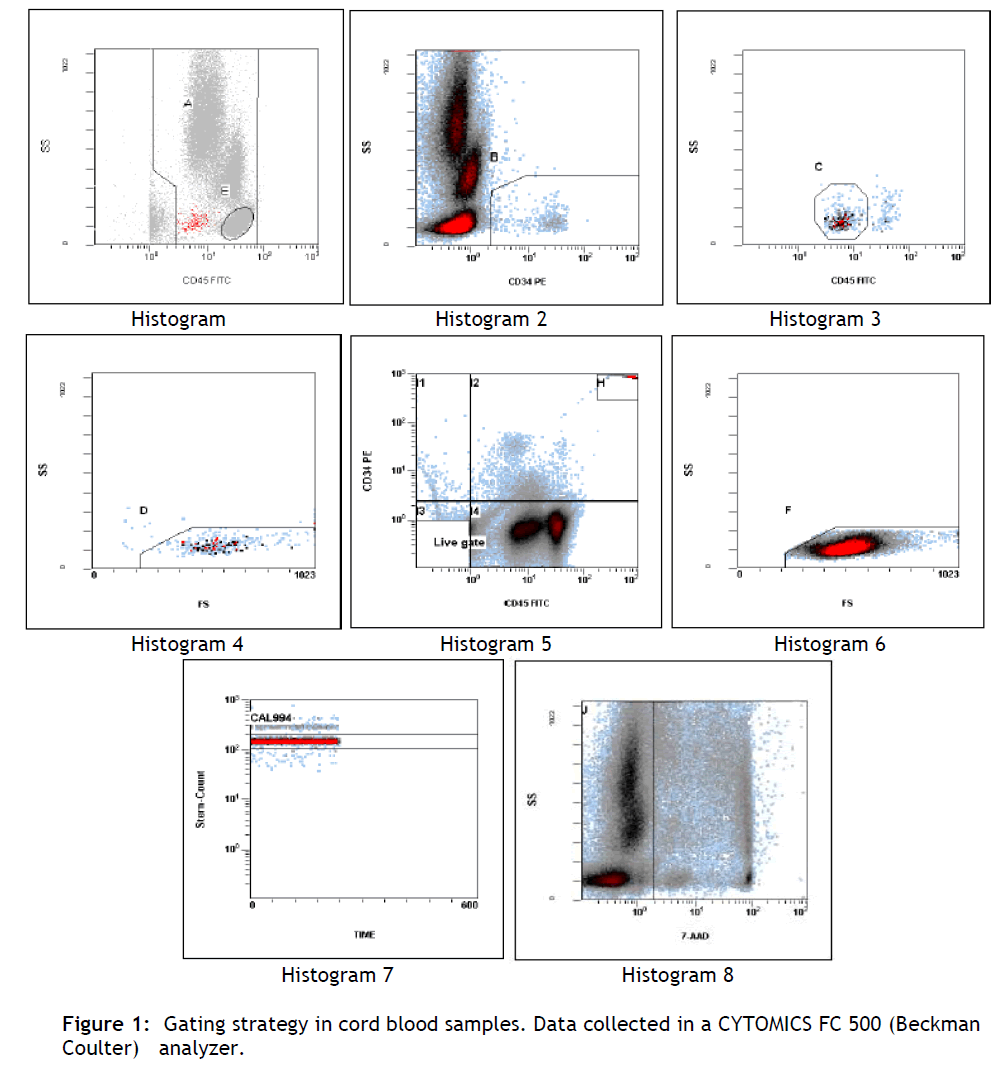
Figure 1: Gating strategy in cord blood samples. Data collected in a CYTOMICS FC 500 (Beckman Coulter) analyzer.
Acquisition and analysis was performed according to the gating strategy recommended by the manufacturer and by using the proprietary software, which in our case was performed using the SSPS statistical software.
Statistical analysis:
Data is given as mean±SEM and analysis was performed using non-parametric statistics since data was not normally distributed. Between groups analysis the Friedman test was used, while for paired analysis Wilcoxon test was performed. For correlation analysis the non-parametric Spearman rho was used. Statistical significance was set at p<0.05 adjusted with Bonferroni correction for multiple comparisons.
Results
By incubating duplicate samples of a cord blood with two colour CD45-FITC/CD34-PE reagent we accomplished specific cell surface staining.
The non-specific binding of the CD34-PE monoclonal antibody was assessed by stained with the CD45-FITC/IsoClonicTM Control-PE reagent.
The CD45-FITC/IsoClonicTM Control-PE reagent is a ready-to-use two colour reagents provided in 1.2mL of PBS containing 2mg/mL BSA and 0.1% sodium azide as a preservative. The CD45-FITC/IsoClonicTM Control-PE reagent is a mixture of unconjugated and PE-conjugated (CD34 identical) 581 antibody. The mixture acts as an appropriate, isomorphologic control to check the non-specific binding of the CD34-RE mAb on each and every sample.
In order to distinguish between viable and non-viable cells 7-AAD viability dye used, that binds to accessible base pairs and allows enumeration of the viable cells of interest, CD34+ cells, (see histogram 8).
Flow-CountTM Fluorospheres, as an absolute count reagent, were added and the cells were analyzed by flow cytometry. Flow-CountTM Fluorospheres are supplied as a bead suspension that necessitates careful handling to ensure accuracy. Each fluorospere contains a dye that has a fluorescence emission range of 525nm to 700nm when excited at 488nm.The Flow-CountTM Fluorospheres assayed concentration (fluorospheres/μL) is derived from multiple replicate analyses using a COULTER particle size analyzer.
Histogram 1: displays viable events from region J (7-AAD negative). Positioned region A to include all CD45+ events (leukocytes) while excluding CD45- events. Positioned region E to include only lymphocytes.
Histogram 2: displays events from region A and J. Adjusted region B to surround CD34+ HPC events. Histogram 3: displays events from A and B and J. Adjusted region C to include cells forming a cluster with characteristic CD34+ HPC. Histogram 4: displays events from A and B and C and J. Lymph/Blast region D identifies a cluster of events meeting all the fluorescence and light scatter criteria of ISHAGE Guidelines for CD34+ HPC. Histogram 5: displays all events. This histogram is useful to visualize the lower limit of CD45 expression within the CD34+ events. Histogram 6: displays events from E and J regions. Histogram 7: displays events from region H. Region J encloses the Stem-Count Fluorospheres singlet population. Histogram 8: displays events from region A. Viable events are included within region J.
The number of CD34+ HPC is calculated as the average of the region D statistical results (see figure 1), for the two replicate samples (45/34/7-AAD # 1 and 45/34/7-AAD # 2). TheCD34+ HPC results are considered valid if the results obtained by the CD45-FITC/IsoClonic Control-PE reagent are less than 10% of the CD34+ HPC results. The value obtained with the 45/CTRL/7-AAD control tube must represent less than 10% of the average value obtained from the tests tubes to validate the results.
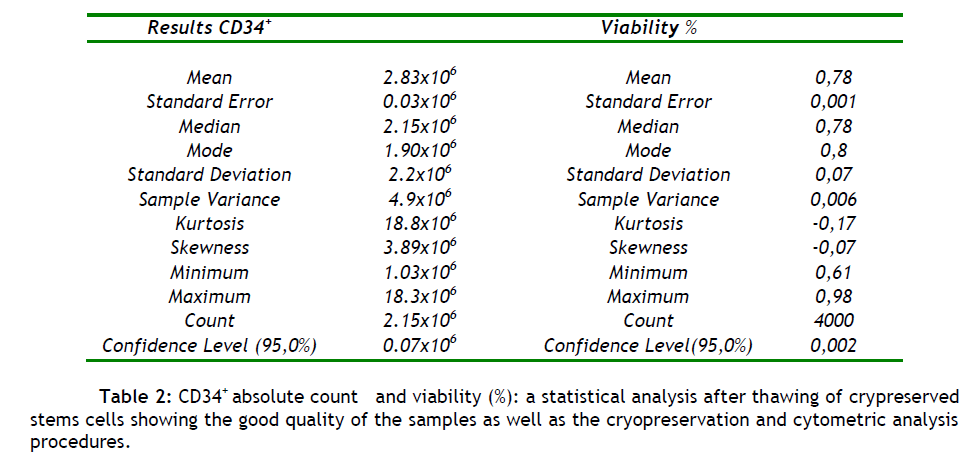
The mean number of CD34+ cells (n = 4000) harvested after thawing of cryopreserved stem cells was 3.0x106 cells/μL with mean cell viability 78% (see tables 2). The data shows the high quality of samples preparation and storage conditions, and also confirms the accuracy and importance of flow cytometry as a quality control technique, for our lab assessing CD34+.
The time group analysis of Flow Cytometry showed that the number of CD34+ cells did not significantly differ between groups (Friedman test p=0.455 n.s.). Consistently the percentage of viability of WBC during time did not significantly alter (p=0.315), (see table 3 and figure 2).
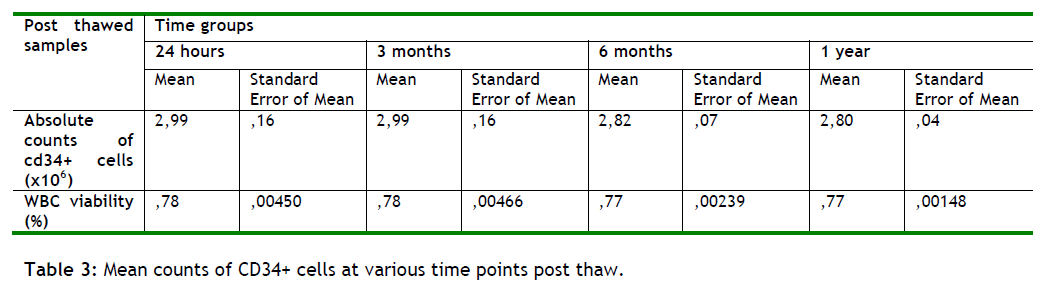
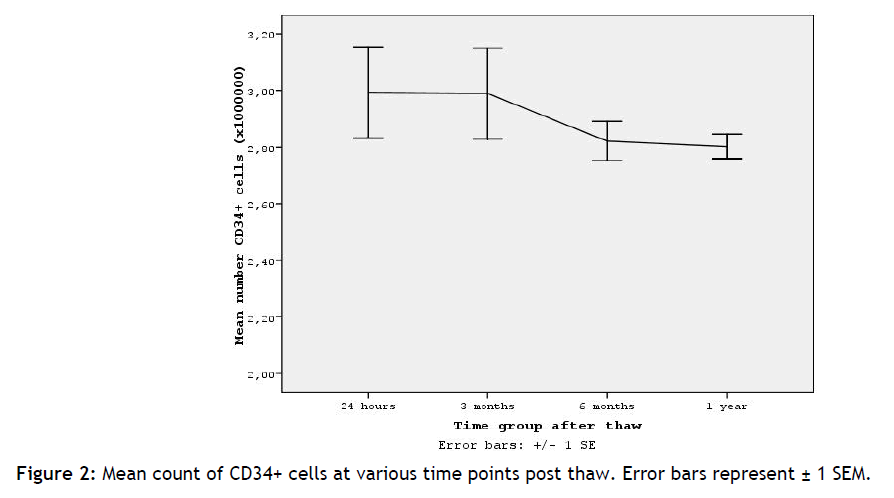
Figure 2: Mean count of CD34+ cells at various time points post thaw. Error bars represent ± 1 SEM.
In order to examine the differences in WBC, between the haematology analyzer and the Flow Cytometry, the counts of WBC from the same sample was compared. The measurements from the haematology analyzer was taken immediately after sample process while the Flow Cytometry counts concerned the same sample at various time points after thawing(24 hours, 3 months, 6 months and 1 year).
It was found that Flow Cytometry gave less absolute WBC count from the haematology analyzer in all time point evaluated(p<0.001), (table 4 and figure 3). The mean difference in WBC counts between the two methods ranged from 0.82 to 1.23. From these results we could conclude that there is not a significant difference between the two methods.
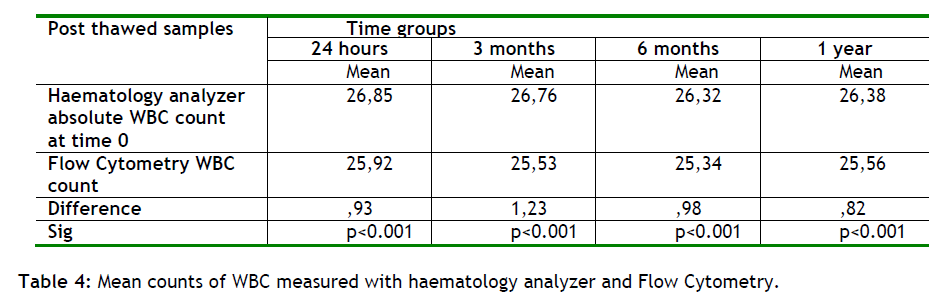
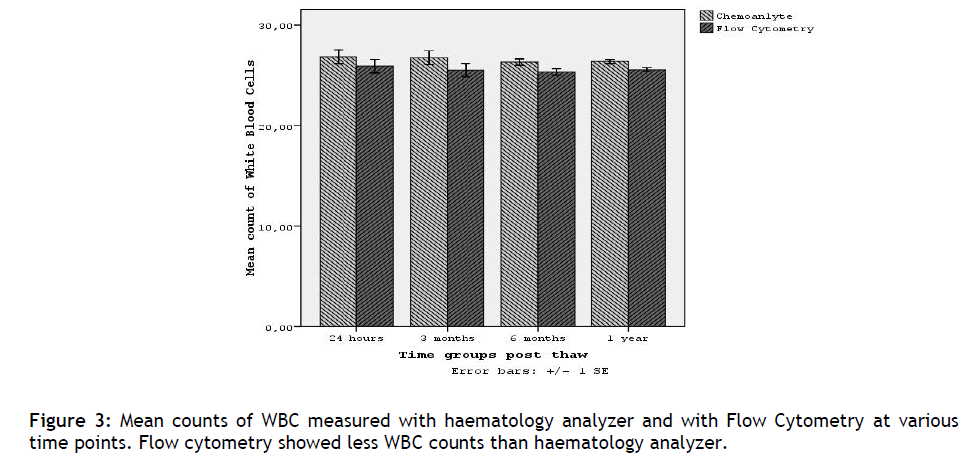
Figure 3: Mean counts of WBC measured with haematology analyzer and with Flow Cytometry at various time points. Flow cytometry showed less WBC counts than haematology analyzer.
Therefore the comparison between the WBC derived from a haematology analyzer and the WBC from the flow cytometer (see table 4), shows the accuracy and efficacy of the technique and can be used as a quality control procedure in our lab.
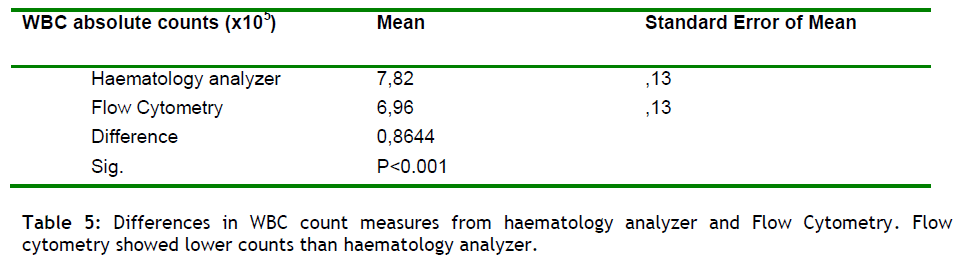
Regarding to the WBC absolute count between the two techniques the Flow Cytometry revealed lower counts of WBC than haematology analyzer (p<0.001). From these results we also could conclude that there is not a significant difference between the two methods.
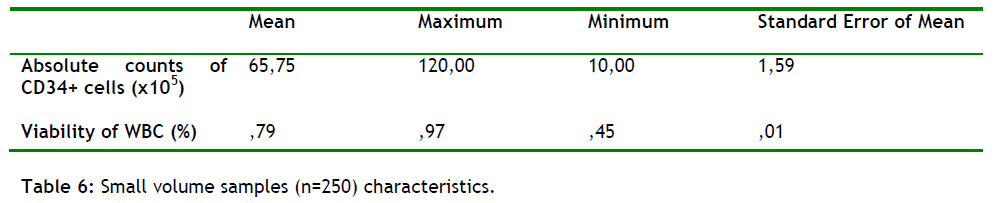
In this study we also aimed to prove the correlation between low counts of WBC and low amounts of CD34+ present in the sample. The conclusion from the comparison between samples (n = 250) with low counts of WBC but very good amount of collection volumes shows a very strong correlation between them in the 95% of the samples.
Samples starting with low WBC will give low amount of CD34+ present in the sample (see table 6). The WBC were also compared at post-freeze (using a haematology analyzer), and at thawed stage (using the flow cytometer).
All samples (n = 250) tested gives us very high reproducible validation rates which prove the precision of the flow cytometric method but also the accuracy of the storage and preparation conditions. This conclusion needs further investigation about the reasons of happening.
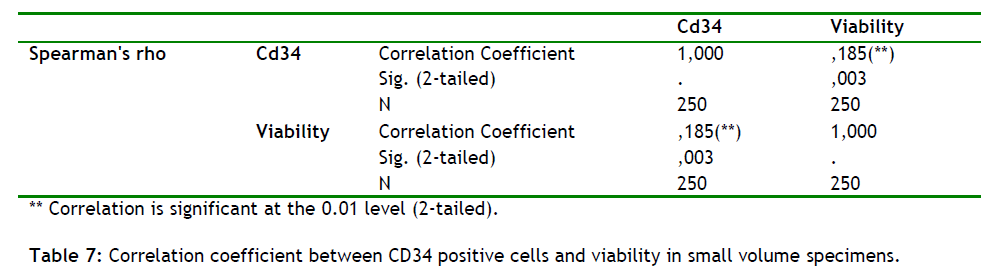
In small volume samples (low values of WBC will give low counts of CD34 +) Flow Cytometry analysis showed that there is a correlation between the viability of WBC and absolute counts of CD34+ cells. Indeed as the absolute counts of CD34+ cells increases the viability increases (spearman rho correlation coefficient 0,185, p=0.003), (see table 7).
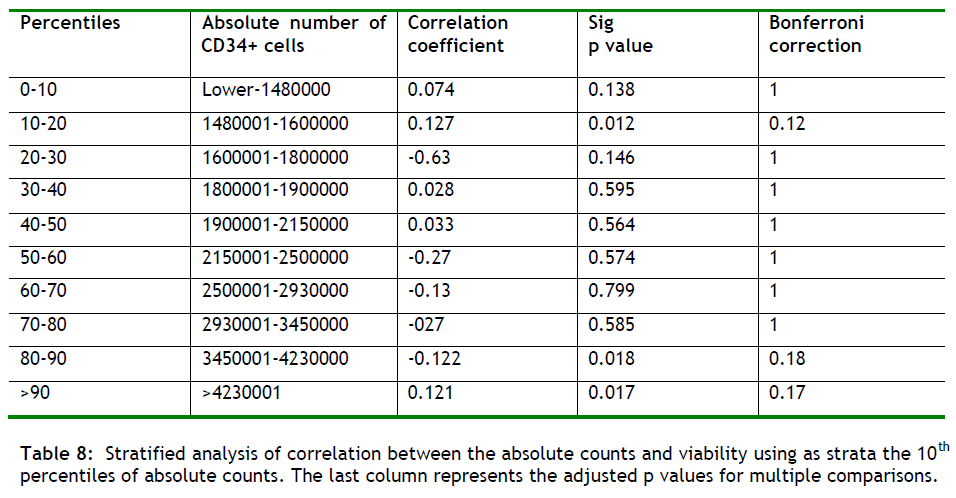
In order to investigate whether the correlation between WBC viability and CD34+ absolute count is seen in adequately large samples (>1 million cells) we performed correlation tests in larger volume samples. We performed a stratified analysis using as strata the 10 percentiles of the absolute number of CD34 +cells and then performed correlation tests in each step. As seen in the table below (table 8) the estimated correlation between the number of CD34+ cells and viability is lost in large volume samples.
Discussion
A chronology of haematopoietic progenitor cells (HPC) scientific reports shows early studies relied upon nucleated cell counts for quantification. Subsequent studies involving colony forming unit (CFU) assays provided rough approximations of progenitor cell populations [21]. The time intensive CFU assays were followed by the discovery of the CD34+ cell surface molecule. CD34+ was found to be present on the surface of early haematopoietic cells and haematopoietic colony-forming cells in bone marrow and peripheral blood [22,23]. The widening use of stem cell mobilization means that a method that accurately and rapidly quantifies CD34+ cells is much needed. Such a method is the flow cytometry [23-25].
With a flow cytometer designed to provide absolute counts, we directly measured the actual number of CD34+ cells present (x10^6 cells/μL) in the sample and their viability (%) (Table 2). In table 2 we show the good quality of the samples collection as well as the cryopreservation and cytometric analysis procedures. This conclusion refers to the mean number of absolute CD34+ counts which was found to be 2.83x10^6 (n = 4000) when the accepted value for a transplantation is 1.00x10^6 cells per kilogram of body weight [25,26] . Also the mean viability value was 77.5% (n = 4000) which shows the good cryopreservation and storage conditions of the samples (table 2).
Viability was also checked by triptan blue exclusion method (n = 4000), and was found to be 82% on average (data not show). These results can serve as good quality control markers for our method.
Referring to tables 3 and figure 2 we show that the mean absolute counts of CD34+ cells present in the samples, in each time group, as well as their viability fluctuating with +/ - 1, suggesting that as time passes the samples absolute counts and their viability remains stable. This conclusion is very promising for the future but more studies in longer periods of time must be done in order to make safe assumptions.
Also we show that the actual WBC numbers in post-freeze from a haematology analyzer and at thawed samples from a flow cytometer, in each time group, were comparable (+ / - 1) (table 4 and 5/ figure 3 and 4). It is known that erythroblast are present in high quantities in cord blood samples, therefore could be a factor that affect the WBC counts in a haematology analyzer, but this assumption was not observed. This is happening because of the erythroblast depletion during the cord blood procedure.
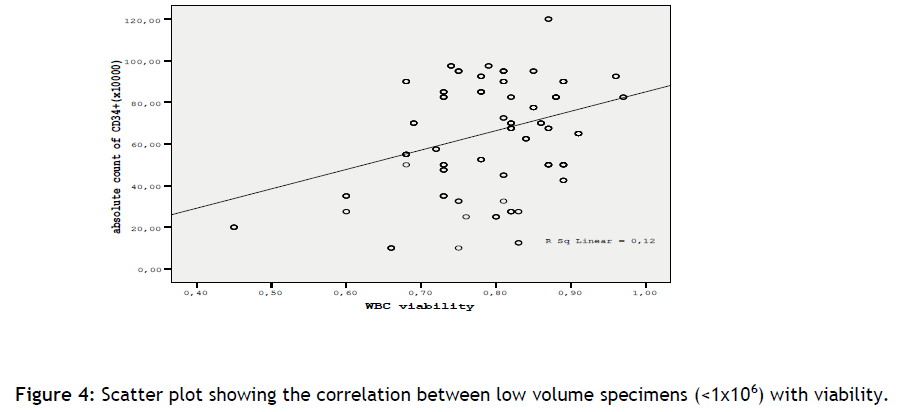
Figure 4: Scatter plot showing the correlation between low volume specimens (<1x106) with viability.
To further elucidate this we did the following: the samples (n = 4000) were assessed for WBC in a haematology analyzer before and after cryopreservation stage. The WBC histograms, (data not show), were compare in the pre- freeze samples and was found that the point of entrance is on the vertical axes, below 35Fl, clearly proving the WBC interference. In the post freeze samples the point of entrance was in the axes cross point which shows that the WBC interference was absent in those samples.
To further investigate the erythroblast interference we used CD71 antibody in stem cells counts in order to find the percentage of remaining erythroblasts in our samples.
CD71 is a single-passtype-2 671 of disulfide-bonded homodimer glycoprotein. The CD71 antibody recognizes the transferring receptor, which is essential for iron transport into proliferating cells. Is expressed on marrow stromal cells (MSCs) from bone marrow, on activated T and B lymphocytes, macrophages, and all proliferating cells. Is also present on reticulocytes and erythroid progenitors in fetal liver, cord blood, and peripheral blood, yet it is lost as these differentiate to mature erythrocytes. The CD71 antibody can be used to identify and enumerate CD71+ cells by flow cytometry.
We found that the amount of erythroblasts present in the sample is 5% (+ /- 1). This finding suggests that the differences between the flow cytometer and the haematology analyzer could not exceed this limit. This verifies our initial observation of a precise correlation between the flow cytometer and the haematology analyzer (table 4 and 5/ figure 3 and 4).
We also found that low counts of WBC will give low counts of CD34+ cells present in the sample (table 6). All samples, (n = 250), start with a collection volume of >60-120ml and after the cytometric analysis gives us a mean viability value of 79% suggesting a good quality of the sample as well as the procedures of cryopreservation and the flow cytometry. This founding suggesting a strong correlation between low values of WBC and low CD 34+ counts.
Also we found that in small volumes samples there is a strong correlation between the viability of WBC and absolute counts of CD34+ cells (table 7), which shows that as the absolute counts of CD34+ cells increases the viability increases as well. Because of this findings and in order to see if there is a correlation between WBC viability and CD34+ cells absolute counts in adequate large samples (> 1million cells), we performed a statistical analysis (n = 4000/time groups, table 7) and a correlation test in each step and was found that the number of CD34+ cells and the viability is lost in the large volume samples. This assumption could help us in the future transplantation procedures.
In summary, cryopreservation and processing of autologous stem cells collection, assessing CD34+ cell viability and absolute counts, found to remain the same during periods of time. Measuring CD34 +cell viability allows us the quality of control assessments of stem cells processing.
The isolation of stem cells offers the promise of a remarkable array of novel therapeutics. Biological therapies derived from such cells, (through tissue regeneration and repair as well as through the targeted delivery of genetic material), are expected to be effective in the treatment of a wide range of medical conditions.
Conditions such as cancer (the 1st transplant from autologous cord blood stem cells has been reported by an American team in a 3rd year old girl and after 3 years is healthy and with no sign of disease, Paediatrics: volume 119, number 1, January 2007), and birth defects are due to abnormal cell division and differentiation. An understanding of the mechanisms that dictate these conditions will be required to develop strategies for therapy.
Conclusion
Cryopreservation and processing of autologous stem cells collection, assessing CD34+ cell viability and absolute counts, found to remain the same during periods of time. Measuring CD34 +cell viability allows us the quality of control assessments of stem cells processing.
Stem cells have great promise for use in regenerative medicine and cell therapy in the future.
3346
References
- Givan AL. Principles of flow cytometry: an overview. Methods Cell Biol. 2001; 63:19-50.
- Brown M, Wittwer C. Flow cytometry: principles and clinical applications in haematology. Clin Chem. 2000; 46:1221-9.
- Stewart CC. Multiparameter flow cytometry. J Immunoassay. 2000; 21:255-72.
- Berardi AC, Wang A, Levine JD, Lopez P, Scadden DT. Functional isolation and characterization of human heamatopoietic stem cells. Science 1995; 267: 104-108
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell 2001; 105:829-841.
- Watt FM, Hogan BL. Out of Edem: stem cells and their niches. Science 2000; 287:1427-1430.
- Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 1995; 11:35-71.
- Junko F, Takako K, Motoki E, Kazuo O. Direct Measurement of CD34+ Blood Stem Cell Absolute Counts by Flow Cytometry. Stem Cells, 1998; 16, 4: 294-300.
- Quesenberry P, Levitt L. Heamatopoietic stem cells. N. Engl. J. Med. 1979; 3001: 755-760.
- Span rude GJ. Biological and clinical aspects of heamatopoietic stem cells. Ann. Rev. Med. 1994; 45: 93-104.
- Krause DS, Fackler MJ, Civin CI, May WS. CD34+: Structure, biology, and clinical utility. Blood 1996; 87: 1-13.
- Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulating of CD34+ haematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: Enhancement by intravenous recombinant human granulocyte-macrophage colony-stimulating factor. Blood 1989; 74: 1905-1914.
- Brenner MK, Rill DR, Moen RC. Gene making to determine whether autologous marrow infusion restores long-term haematopoiesis in cancer patients. Lancet, 1993; 6: 1134-1137.
- Oscar F, Joan G, Jordi P. Flow cytometry counting of CD34+ cells in whole blood. Nature Medicine, 2000; 6: 833-836.
- Scheffold A, Kern F. Recent developments in flow cytometry. J.Clin.Immunol. 2000; 20: 400-7.
- Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J. Hematotherapy, 1996; 5: 213-226.
- Gratama JW, Keeney M, Sutherland R. Enumeration of haematopoietic stem and progenitor cells. Current Protocols in Cytometry, 1999; 6.4.1-6.4.22.
- Sutherland DR, Keating A. The CD34+ antigen: structure, biology and potential clinical applications. Hematotherapy, 1992; 1: 115-129.
- Keeney M., Chin-Yee I., Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cells counts based on the ISHAGE guidelines. Cytometry, 1998; 34: 61-70.
- Civin CI., Trischmann TM, Fackler MJ, Bernstein ID, Buhring HJ, Campos L, et al. “Report on the CD34 cluster workshop” in Leucocyte Typing IV, White Cell Differentiation Antigens, Oxford Univ. Press, 1989; 818-825.
- Papassavas AC, Gioka V, Chatzistamatiou T, Kokkinos T, Anagnostakis I, Gecka G, et al. A strategy of splitting individual high volume cord blood units into two half subunits prior to processing increases the recovery of cells and facilitates ex vivo expansion f the infused haematopoietic progenitor cells in adults. International Journal of Laboratory Hematology, 2007; 1-9.
- Brocklebank AM, Sparrow RL. Enumeration of CD34+ cells in cord blood: a variation on a single-platform flow cytometric method based on the ISHAGE gatting strategy. Cytometry 2001; 46:254-261.
- Satherland DR, Keeney M., Chin-Yee I, Anderson L, Nayar R. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. Journal of Hematology 1996; 5:213-223.
- Barnett D, Janossy G, Lubenko A, Matutes E, Newland A, Reilly JT. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Clin. Lab. Haem. 1999; 21:301-308.
- Copelan EA. Haematopoietic stem cell transplantation. N Engl. J Med 354: 2006; 17: 1813-26.
- Cutler C, Antin J.H. Peripheral blood steam cells for allogenic transplantation: a review. Stem Cells 2001; 19: 108-17.



















