Keywords
Second-hand smoke; Environmental tobacco smoke; Asthma; Wheezing; Urinary nicotine/cotinine
Introduction
Second-hand smoke (SHS) exposure is a serious health hazard for children [1] as it has been causally linked to increased risk of sudden infant death syndrome, respiratory tract and middle ear infections, smoke-caused coughs, wheezing and asthma. According to World Health Organization (WHO) almost half the children worldwide are exposed to second-hand smoke with the highest level of exposure observed in Europe (71.5%) [2].
According to the 2006 US Surgeon General report there is no safe level of SHS exposure [3]; even brief exposure can be harmful. Children may be exposed to second-hand smoke practically anywhere (public places, transportation, family car etc.,) but the primary source of SHS exposure is in their home [4]. Greece is among the countries that face a serious smoking problem [5] with an estimated 40% of the adult population being current daily smokers. This high percentage of smokers highlights the children SHS exposure problem in Greece.
Asthmatic children are in particular danger as SHS exposure lead to more severe symptoms and more frequent asthma exacerbations [6]. Not surprisingly, avoiding SHS exposure has always been recommended as an important component of asthma management [7]. Although parents describe SHS avoiding practices at home many children, even those with significant asthma, continue to experience SHS exposure [8]. This finding is mostly a questionnaire outcome without proper laboratory verification.
The purpose of this study was to explore parental smoking habits in homes of children with bronchial asthma/wheezing disorders and healthy children and to verify questionnaire outcomes by laboratory testing.
Materials and Methods
Study population
This case-control study was conducted during September 2014 to September 2015. The cases group included 51 consecutive outpatient children with bronchial asthma/ wheezing syndrome who were being treated at the Pediatric Pulmonology Unit of 2nd and 4th Pediatric Department of Aristotle University of Thessaloniki, Greece. Wheezing syndrome (whether viral or multi-trigger) was defined as having had at least 3 previous wheezing episodes (continuous, high-pitched sound from the chest, coughing, difficulty breathing) by the age of six years old [9]. Children with bronchial asthma were considered those aged ≥ 5 years old and met GINA 2015 criteria for asthma diagnosis (history of respiratory symptoms, clinical findings, lung function tests confirming asthma) [7].
The control group consisted of 39 healthy children with no respiratory tract or chronic disease who proceeded to outpatient units for routine check-up. Inclusion criteria were 1) 5-18 years old aged so that they can have lung function testing 2) no history of asthma/wheezing disorders 3) no history of recent respiratory tract infection.
Exclusion criteria were major metabolic dysfunctions and severe chronic diseases or pathologic conditions imitating asthma/wheezing (chronic respiratory system infections, rhinitis, tuberculosis, congenital heart-diseases, bronchopulmonary dysplasia and congenital anatomic defects in respiratory tract, cystic fibrosis, gastroesophageal reflux and immunodeficiency). Children who were active smokers were also excluded as well as children from a single parent home.
Cases and controls had no history of prematurity and were simultaneously recruited in the study. One parent active smoker was prerequisite for both groups.
All children were submitted in complete clinical examination and spot urine sample collection.
Clinical examination and tests were performed by the same qualified person who also fulfilled questionnaire after personal interview with either parent.
According to the study protocol child and family history of asthma/wheezing and allergies was recorded. Socioeconomic variables included parents’ education, occupation, house and car ownership and number of persons living in the house. Persons living in the house variable was divided into ≤ 4 representing the traditional four-membered Greek family (mother, father, two children) and >5 for extra persons living in the house (siblings, grandparents, other caregivers, etc.,). Parents educational level was subdivided into primary, secondary and university graduate.
Definitions
Smoking (for active smokers) was defined as consumption of at least one cigarette per day. Cigarette consumption was subdivided into <5 cigarettes per day, 5-19 and ≥ 20 cigarettes per day.
Second hand smoke (SHS) was defined as a mixture of side stream smoke from the burning end of a cigarette and exhaled mainstream smoke; second-hand smoking or exposure to SHS is involuntary inhalation of cigarette smoke by non-smokers.
Third hand smoke (THS) was defined as residual tobacco smoke pollutants that remain on surfaces and in dust after tobacco has been smoked, are re-emitted into the gas phase, or react with oxidants and other compounds in the environment to yield secondary pollutants [10].
Smoking in the house was considered if any parent smoked inside any room of the house while smoking on the balcony/ veranda, courtyard, rooftop, was considered as smoking outside the house. This was considered as complete restrictions of smoking at home. Partial restrictions were considered if parents smoked inside the house but only in a specified room and no restrictions were considered for the families with no bans on where smoking occurred in the household.
Smoke exposure
Second-hand smoke exposure was assessed on the basis of parent self-report as documented by the questionnaire and urinary nicotine and cotinine measurements in children.
The questionnaire inquired about the following information: parental smoking behavior, smoking restrictions at home, daily cigarette consumption, number of persons exposing children to passive smoking at home.
For urine analysis, spot urine sample was collected from each child at Pediatric Clinics settings and stored at -20°C. Gas chromatography tandem mass spectrometric (GC-MS) was the method chosen for detection and quantification of urine nicotine and cotinine. 1ml dichloromethane was selected for the extraction of 0.5 ml of urine. Analysis was performed on an Agilent Technologies 7890A GC, combined with a 5975C inert XL EI/CI MSD with Triple-Axis Detector (Agilent Technologies) equipped with a CTC auto sampler. Suitable chromatographic conditions were found for the rapid and accurate determination of nicotine and cotinine. The method was validated to meet criteria for application in bio analytical laboratory. Injection of a 1 μl was done in GC/MS and SIM (selected ion monitoring) analysis was performed with the following ions (amu m/z): 162 (quantifier ion) 84, 133, 161 qualifier ions for nicotine and 176 (quantifier ion) 98, 118, 119, 147 qualifier ions, for cotinine. Cotinine D3 was used as internal standard with 101 amu as the quantifier ion. The retention time (Rt) was for nicotine 9.13 min, for cotinine 11.63 min and for the IS 11.61 min. The duration of the GC-MS analysis was 25 minutes. The method showed linear dynamic range from 10 ng/ml to 400 ng/ml. The lab tests were performed with no knowledge whatsoever of the questionnaire responses. Cotinine levels were calculated in terms of ng/mL and subdivided into low (<5 ng/ml) [11] and ≥ 10 ng/ml SHS exposure.
Ethics
All parents were well informed for the participation of the family in the survey and written consent was given. The study was approved by the Aristotle University of Thessaloniki Ethics Research Council.
Statistical analysis
Data was analyzed in the Statistical Package for the Social Sciences 20.0 (SPSS Inc., Chicago, IL, USA). Characteristics of children and their families participating in the study were calculated in groups (cases-controls) as well as parental smoking habits and nicotine/cotinine levels in children’s’ urine. Cotinine levels were divided into three categories (as mentioned above) by creating an ordinal variable that was progressively increasing.
Descriptive measurements were used to define the characteristics of children in the study. When variables were normally distributed t-test and ANOVA were used to test the associations among the variables analyzed in two groups. Otherwise Mann-Whitney και Kruscal-Wallis non-parametric tests were performed. Test of normality was conducted using Kolmogorov-Smirnof and Shapiro-Wilk test as well as histograms, P-P και Q-Q graphs. To analyze trends in percentages, Chi-square tests were performed. Relationships with a p-value (p) ≤ 0.05 were considered as statistically significant. In results’ presentation continuous variables are demonstrated as mean ± standard deviation whereas qualitative variables are depicted with the use of frequencies. Parental smoking habits are also shown in bar charts.
Results
This case-control study monitored 90 children aged 1.5-17 years old and their parents. They were subdivided in two groups; asthmatic children (cases) included 51 children with average age 7.31 ± 3.81 years, and healthy children (controls) with average age 9.46 ± 3.46 years. Boys to girls’ ratio were 1.83 and 1.16 in cases and controls respectively. Table 1 shows the overall demographic characteristics of children and their families recruited in survey. Cases were slightly older than controls and boys outnumbered girls in both groups. Significant more mothers in cases were university graduates and most parents’ education was secondary but overall educational level between two groups (secondary education for at least one parent) was not significant. Most children lived in the traditional Greek four membered families owning the house and a car. As expected more cases than controls had personal and family history of asthma/allergies.
Table 1 Demographic characteristics of the surveyed population.
| |
N(51) |
Cases |
N(39) |
Controls |
p-value |
| Age |
|
7.31 ± 3.81 y |
|
9.46 ± 3.46 |
0.007 |
| Gender |
| Male |
33 |
64.70% |
21 |
53.80% |
0.297 |
| Female |
18 |
35.30% |
18 |
46.20% |
| Mothers age |
|
35.7 ± 4.85 |
|
37.87 ± 7.17 |
0.092 |
| Fathers age |
|
38.86 ± 5.34 |
|
41.74 ± 7.45 |
0.036 |
| Mothers age at pregnancy |
|
28.55 ± 4.51 |
|
28.3 ± 5.45 |
0.812 |
| Mothers education |
| Primary |
7 |
13.70% |
10 |
25.60% |
0.025 |
| Secondary |
31 |
60.80% |
27 |
69.20% |
| University |
13 |
25.50% |
2 |
5.10% |
| Fathers education |
| Primary |
7 |
13.70% |
10 |
25.60% |
0.332 |
| Secondary |
41 |
80.40% |
27 |
69.20% |
| University |
3 |
5.90% |
2 |
5.10% |
| Residence |
| Urban |
37 |
72.50% |
30 |
76.90% |
0.882 |
| Semi-urban |
9 |
17.60% |
5 |
12.80% |
| Rural |
5 |
9.80% |
4 |
10.30% |
| House ownership |
46 |
90.20% |
36 |
92.30% |
1 |
| Car ownership |
43 |
84.30% |
33 |
84.60% |
0.969 |
| Persons living in the house |
| ≤4 |
38 |
74.50% |
28 |
71.80% |
0.773 |
| >5 |
13 |
25.50% |
11 |
28.20% |
| Diagnosis |
| Bronchial asthma |
| Mild |
33/51 |
64.70% |
|
|
|
| moderate |
Mar-51 |
5.90% |
|
|
|
| Wheezing (viral or multi trigger) |
15/51 |
29.40% |
|
|
|
| Child history of allergies |
29 |
56.90% |
7 |
17.90% |
0.002 |
| Family history of wheezing/allergies |
44 |
86.30% |
16 |
41% |
<0.01 |
In 70% (63/90) of the families surveyed both parents were active smokers with 15.6% (14/90) only mother to be a smoker and in the rest 14.4% (13/90) only the father. Fathers were heavier smokers than mothers (24.01 ± 17.57 cigarettes daily versus 17.12 ± 14.85, p=0.519) with mothers in controls to smoke slightly more than mothers in cases (19.53 ± 18 cigarettes daily versus 15.27 ± 11.75, p=0.508). Otherwise 43.3% of mothers and 75.6% of fathers smoked more than 20 cigarettes daily, the difference being significant (p=0.001). Father’s (Figure 1) and mother’s (Figure 2) daily cigarette consumption was not statistically different between two groups. Rates of mothers and fathers’ daily smokers between two groups were not significant (Table 2).
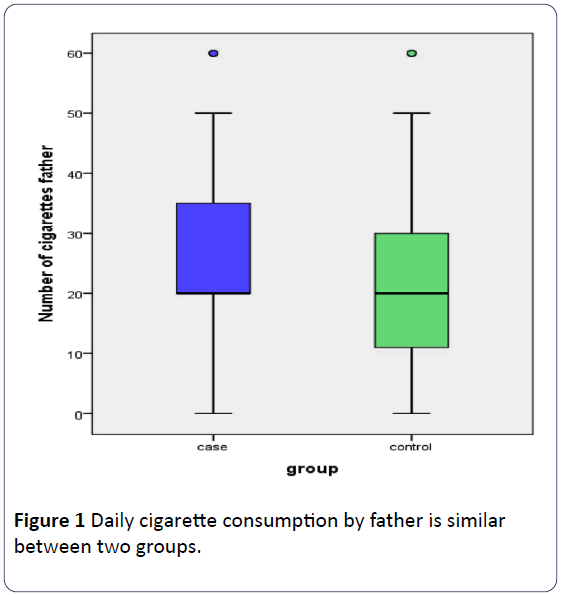
Figure 1: Daily cigarette consumption by father is similar between two groups.
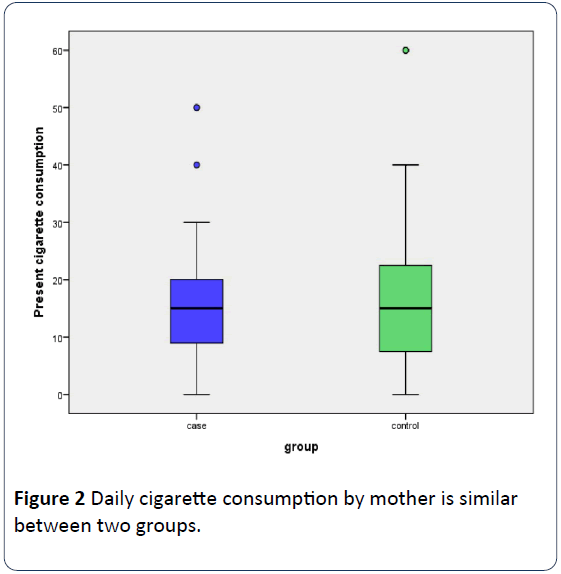
Figure 2: Daily cigarette consumption by mother is similar between two groups.
Table 2 Parental smoking habits at home and SHS exposure of the child.
| |
N |
Cases |
N |
Controls |
p-value |
| Mothers present daily smokers |
43/51 |
84.3% |
34/39 |
87.2% |
0.508 |
| Fathers present daily smokers |
42/51 |
82.4% |
32/39 |
82.1% |
0.97 |
| Persons exposing child to passive smoking |
| Only mother and/or father |
40/51 |
78.4% |
27/39 |
69.2% |
0.291 |
| Other persons than mother and father |
11/51 |
21.6% |
12/39 |
30.8% |
Table 2 also shows similar exposure to parental smoking in both groups and many kids to be exposed to passive smoking by other persons than their parents (family members, parents’ friends, etc.,) especially in controls.
The majority of children in both groups were positive in urine nicotine and cotinine detection with cases’ nicotine detection being significant (Table 3). In quantification, averages of urine nicotine and cotinine were found higher in cases with cotinine slightly not to reach statistical significance. 44.4% of children had <5 ngr/ml urine cotinine concentration and 33.3% had ≥ 10 ngr/ml. Half the children in controls had low SHS exposure (<5 ngr/ml) and more children in cases had ≥ 10 ngr/ml SHS exposure, the results being not significant (Table 4).
Table 3 Urine nicotine and cotinine measurements in children.
| |
N |
Cases |
N |
Controls |
p-value |
| Positive in urine nicotine (qualitative) |
45/51 |
88.24% |
28/39 |
71.79% |
0.026 |
| Positive in urine cotinine (qualitative) |
50/51 |
98% |
35/39 |
89.74% |
0.162 |
| Urine nicotine (quantitative) |
|
3.49 ± 3.8 |
|
2.52±2.38 |
0.188 |
| Urine cotinine (quantitative) |
|
8.6 ± 7 |
|
6.26±5.67 |
0.069 |
Table 4 Urine cotinine graduation in children.
| |
N |
cases |
N |
controls |
p-value |
| <5 ngr/ml |
20/51 |
(39.2%) |
20/39 |
(51.3%) |
0.254 |
| 5-10 ngr/ml |
12/51 |
(23.5%) |
8/39 |
(20.5%) |
0.775 |
| ≥10 ngr/ml |
19/51 |
(37.3%) |
11/39 |
(28.2%) |
0.344 |
Table 5 shows home smoking restrictions in both groups. There was statistical significance for the place mothers smoke, with the 90.7% of mothers in cases reporting smoking outside the house but half in the control group (p=0.024). For the same question 71.43% of fathers in cases reported smoking outside the house versus 59.37% of controls (p=0.145). Partial bans in smoking were more likely to be imposed in cases’ homes but the overall smoking restrictions at home between two groups didn’t reach statistical significance and were not correlated to mothers’ (p=0.939) or fathers’ (p=0.853) educational level. Home smoking restrictions affected urinary cotinine levels (p=0.02) and more specifically in households where full restrictions were imposed urine cotinine levels of children were more likely to be lower compared to those of children in households where there were no restrictions at all (p=0.015). Even if partial restrictions were imposed still urine cotinine levels were more likely to be lower compared to urine measurements of children in households with no restrictions at all (p=0.044). Though, there was no significance in urine cotinine levels whether full or partial smoking bans were applied (p=0.512). When controlling separately for cases and controls on smoking restrictions at home, urinary nicotine (p=0.306 for cases and p=0.542 for controls) and cotinine (p=0.39 for cases and p=0.07 for controls) medians were not statistically different.
Table 5 Smoking restrictions at home.
| |
N |
Cases |
N |
Controls |
p-value |
| Smoking restrictions |
| Full |
19/51 |
37.25% |
13/39 |
33.4% |
0.329 |
| Partial |
4/51 |
7.85% |
9/39 |
23% |
| No restrictions |
28/51 |
54.9% |
17/39 |
43.6% |
| Mothers smoking outside the house |
39/43 |
90.7% |
14/34 |
41.18% |
0.024 |
| Fathers smoking outside the house |
30/42 |
71.43% |
19/32 |
59.37% |
0.145 |
Discussion
According to both questionnaires and urinary nicotine and cotinine measurements this study shows that parental smoking habits between families of children with asthma/ wheezing disorders diagnosis and their healthy peers are not statistically significant. Several studies can be found in literature confirming SHS exposure of children with asthma/ wheezing and its detrimental effects on respiratory health [11] but few confirm questionnaire outcomes by laboratory testing. Our study is the first one to our knowledge in Greece immediate comparing parental smoking habits in homes with children with and without asthma/wheezing and SHS exposure confirmed by laboratory tests. Laboratory confirmation is essential as there is often a discrepancy in outcomes between questionnaires and laboratory tests, well analyzed subsequently.
According to questionnaires maternal and paternal smoking rates in our study were similar in both groups with more than 80% of mothers and fathers to be present daily smokers. In the large VESTA French study smoking habits were not significant between families with children with and without asthma either. More specific the proportion of smokers among parents did not differ between children with and without asthma, whereas current smoking habits tended to be more important among children with asthma, the difference being significant for mothers [12]. Literature has focused primarily on mothers’ smokers in estimating SHS in children because mothers than fathers spend more time with their children, especially the youngest, and it seems that maternal rather than paternal smoking has a stronger effect on children’s SHS exposure in both healthy [13] and asthmatic children [14]. SHS depend on several maternal smoking characteristics such as socioeconomic and educational status, with most significant for Greek mothers the number of daily cigarette consumption [15] (no significance between two groups in our study). Significantly more mothers in cases had University education. This finding is in contrast with international reports [16] where less educated mothers are more likely to smoke and expose their children to SHS.
Fathers in both groups were heavier smokers than mothers. Previous work has indicated that for respiratory illness in infancy, the effect of father’s smoking in households where the mother does not smoke is statistically significant [17]. Blackburn et al. found that in households where both parents smoke, fathers' tobacco consumption was significantly higher than in households where only the father smokes [18]. This suggests that we have to focus on the interaction between parents rather than isolating mothers' or fathers' smoking behavior.
A large population based study conducted in Greece [19] showed a 63% of households with preschool children, had at least one smoker parent and in 26% both parents were found to be current smokers with 19.6% of them smoking in the presence of their children. In our study 66.7% of parents in cases and 74.4% in controls were both active smokers. Males were also found to be heavier smokers, as in our study, averaging 23 (± 12) cigarettes/day compared to the 15 (± 9) cigarettes for females. Overall smoking prevalence among adults with preschool children was estimated at 44% (52% of fathers and 36% of mothers) which is similar to rates listed in literature for children with asthma [20]. Another Greek study [15] has also reached same rates of parental smoking and showed that the average person’s exposing children to SHS in the house in Greek families is 1.5 ± 0.5. In our study one in five children in cases and almost one in three in controls were exposed to SHS by more people than parents (Table 2). Whether one or both parents smoke, parental smoking at home is the single most important source of passive exposure in childhood [21], although other family members, care givers, visitors or friends may also contribute to the smokiness in the home [21]. Even if some studies conclude that parents alter their smoking behavior after asthma symptoms debut [22], most show that children with asthma continue to be exposed to SHS [23] and in some cases may be heavier exposed than their healthy peers.
According to laboratory urine tests in our study (Table 3) even more children proved to be SHS exposed than unveiled by questionnaires. Nicotine qualitative detection was significant between two groups whereas cotinine quantitative measurement was borderline not significant over cases. Nicotine and cotinine are usually used to assess SHS exposure in both active and passive smokers with cotinine to be preferred due to its longer half-time [24]. This may be the explanation in our study for the children found positive in cotinine but not in nicotine.
An objective measurement of SHS in children is essential as SHS prevalence is likely underestimated by questionnaire data [12] as shown in our study. Additionally, most parental questionnaires cannot discriminate accurately between unexposed children and mildly exposed. In our study 37.3% of cases and 28.2% of controls had urinary cotinine measurements ≥ 10 ng/ml (Table 4). There are studies concluding that children with asthma show higher cotinine concentrations in hair and urine compared to children without asthma [25] but this is probably due to higher exposure of children with asthma to SHS rather than differences in metabolism [26]. Low concentration of urinary cotinine does not diminish SHS health hazards as metabolites of tobaccospecific lung carcinogens were found in the urine of children with low exposure to SHS [11]. Low-levels of SHS exposure may also affect lung function, possibly in a dose-dependent manner [23] and as proved correlate with an increase in inflammatory biomarkers, at least in children with ICS treated asthma [27]. Finally, nicotine biomarkers may be more predictive of asthma exacerbation frequency while caregiver- reported household smoking is not.
Half families in both groups had no smoking restrictions at all in their homes. One third had full restrictions and more controls than cases reported partial restrictions, such as smoking near the window or fireplace or in the kitchen with the ventilator on. Significant more mothers and fathers in cases reported to smoke outside the house but overall smoking restrictions did not differ between two groups (Table 2). Smoking restrictions at home are substantial as if imposed strictly urine cotinine levels in children diminish as proved in the study of Spencer et al. [28]. The same study shows that less strict measures appear to have no effect on infant smoke exposure. In concordance with those findings Wakenfield et al. who investigated smoking habits in families with asthma diagnosis on a child, reported that making exceptions to bans on smoking at home measurably undermines the protective effect of a ban and if so the children's urinary cotinine adjusted for creatinine levels were no different from homes in which smoking was allowed in rooms the child rarely frequented [29]. In our study, statistical significance in urine cotinine levels arose for children living in households with full or partial smoking bans contrary to children living in households with no restrictions at all.
Our study, as many others in literature, show that there is a discrepancy in outcomes between questionnaires and laboratory tests. A meta-analysis of studies that validated selfreported smoking behavior with biochemical measurements concluded that self-reports of smoking status are generally acute, especially in student population and in intervention studies [30], but not in special populations such as pregnant women and children. Questionnaire-derived parental measures of SHS exposure have been shown to correlate poorly with biomarkers in the infant or child. In 24 children registered as unexposed to ETS by parental reports Nafstad et al. [31] found detectable levels of urine cotinine and hair nicotine. Low agreement between parental report of child’s exposure and urinary cotinine was found in many studies [12,32]. In a case-control study [33] with asthmatic and healthy children aged 5-7 years old found 69% of cases to be exposed to SHS as measured by salivary cotinine versus 31% by parental reports to questionnaire. The figures from the control group were 50% and 40% respectively. As documented by other studies there is a trend by the parents of children with asthma/wheezing to under report smoking habits [23]. Questionnaires in controls also failed to reveal SHS in this group even though differences were not as apparent as in cases. Brunekreef et al. emphasize that under reporting of ETS exposure by parents of study children varies, and may depend on the instrument used, population studied, age, and symptom status, underlining the need for questionnaire validation in specific study settings [34]. Questionnaires do not account for the possibility of under reporting, due to unrecognized exposure to ETS from babysitters, grandparents, friends, etc. or exposure to third-hand smoking. Even if parents do not smoke in the house, tobacco smoke particulates remain on their hands and clothes and surfaces or even travel indoors through windows, doors and ventilator systems to contaminate children. Although the term THS is relatively new it is worth attending as emerging evidence links THS to several health effects, especially in children [35]. THS is present even if full smoking bans are imposed indoors and urinary cotinine concentrations in children are strongly associated with the house dust concentrations of nicotine at home [22].
Smoking bans in home are difficult to establish and pediatricians should have a key role in approaching smoking families, particularly if there is a child with asthma/wheezing diagnosis. Urinary cotinine has been shown to be an excellent biomarker of recent ETS exposure and correlate with house dust concentrations of nicotine [22] and asthma symptoms [36]. Physicians should investigate on home smoking restrictions and if possible order laboratory test confirmation of passive smoking. Consistent screening and counseling regarding smoke exposure would help to reduce the burden of smoke-related morbidity for all children and especially for children with asthma/wheezing.
The present study has several limitations with most important the small sample size. Secondary, questionnaire didn’t inspect for smoking behavior of other caregivers or visitors at home or exposure outside the house. Cotinine choice as biomarker has inherent limitations as it depends on individual variability in nicotine metabolism.
Conclusion
Based on questionnaires investigating parental smoking habits and children’s urinary cotinine, this study concluded that there are no statistically significant differences in smoking habits of families with children with and without asthma/ wheezing. Greek parents seem to underreport smoking habits. Tobacco smoke is deleterious and pediatricians should be able to perceive children in danger and intervene.
Conflict of Interest
The authors declare no conflict of interest.
20723
References
- Royal College of Physicians (2010) Passive smoking and children. A report of the tobacco advisory group of the Royal College of Physicians. Royal College of Physicians, London.
- Gordon B, Mackey R, Rehfuess E (2004) Inheriting the World: The atlas of children's health and the environment. World Health Organization, Geneva.
- US Surgeon General (2006) The health consequences of involuntary exposure to tobacco smoke: A report of the Surgeon General.
- Shaw A, Ritchie D, Semple S, Turner S, O’Donnell RR, et al. (2012) Reducing children’s exposure to second hand smoke in the home: A literature review. A report by ASH Scotland.
- Vardavas CI, Kafatos AG (2006) Greece's smoking policy: Another myth? Lancet 367: 1485-1486.
- Strachan DP (1998) Parental smoking and childhood asthma: Longitudinal and casecontrol studies. Thorax 53: 204212.
- Irvine L, Crombie IK, Clark RA, Slane PW, Goodman KE, et al. (1997) What determines levels of passive smoking in children with asthma? Thorax 52: 766769.
- Brand PL, Baraldi E, Bisgaard H, Boner AL, Castro-Rodriguez JA, et al. (2008) Definition, assessment and treatment of wheezing disorders in preschool children: An evidence-based approach. Eur Respir J 32: 1096-1110.
- Matt G, Quintana P, Destaillats H, Gundel LA, Sleiman M, et al. (2011) Third hand tobaccos smoke: Emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect 119: 1218-1226.
- Hecht SS, Ye M, Carmella SG, Fredrickson A, Adgate JL, et al. (2001) Metabolites of a tobacco-specific lung carcinogen in the urine of elementary school-aged children. Cancer Epidemiol Biomarkers Prev 10: 1109-1116.
- Callais F, Momas I, Roche D, Gauvin S, Reungoat P, et al. (2003) Questionnaire or objective assessment for studying exposure to tobacco smoke among asthmatic and healthy children: The French VESTA Study. Prev Med 36: 108-113.
- Puig C, Garcia-Algar O, Monleon T, Pacifici R, Zuccaro P, et al. (2008) A longitudinal study of environmental tobacco smoke exposure in children: parental self reports versus age dependent biomarkers. BMC Public Health 8: 47.
- Ehrlich R, Kattan M, Godbold J, Saltzberg DS, Grimm KT, et al. (1992) Childhood asthma and passive smoking: urinary cotinine as a biomarker of exposure. Am Rev Respir Dis 145: 594-599.
- Mantziou V, Vardavas C, Kletsiou E, Priftis K (2009) Predictors of childhood exposure to parental secondhand smoke in the house and family. Car Int J Environ Res Public Health 6: 433-444.
- Thaqi A, Franke K, Merkel G, Wichmann HE, Heinrich J (2005) Biomarkers of exposure to passive smoking of school children: frequency and determinants. Indoor Air 15: 302-310.
- Cook DG, Strachan DP (1999) Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax 54: 357-366.
- Blackburn CM, Bonas S, Spencer NJ, Coe CJ, Dolan A, et al. (2005) Parental smoking and passive smoking in infants: Fathers matter too. Health Educ Res 20: 185-194.
- Vardavas C, Athanasopoulos D, Balomenaki E, Niaounaki D, Linardakis M, et al. (2007) Smoking habits of Greek preschool children's parents. BMC Public Health 7: 112.
- Kumar R, Curtis LM, Khiani SA, Moy J, Shalowitz MU, et al. (2008) A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. J Allergy Clin Immunol 122: 754-759.
- Cook GC, Wincup PH, Jarvis MJ, Strachan DP, Papacosta O, et al. (1994) Passive exposure to tobacco smoke in children aged 5–7 years: Individual, family, and community factors. Br Med J 308: 384-389.
- Willers S, Axmon A, Feyerabend C, Nielsen J, Skarping G, et al. (2000) Assessment of environmental tobacco smoke exposure in children with asthmatic symptoms by questionnaire and cotinine concentrations in plasma, saliva, and urine. J Clin Epidemiol 53: 715-721.
- Valsamis C, Krishnan S, Dozor AJ (2014) The effects of low-level environmental tobacco smoke exposure on pulmonary function tests in preschool children with asthma. J Asthma 51: 685-690.
- Benowitz NL (1996) Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 18: 188-204.
- Willers S, Svenonius E, Skarping G (1991) Passive smoking and childhood asthma. Urinary cotinine levels in children with asthma and in referents. Allergy 46: 330-334.
- Leong JW, Dore ND, Shelley K, Holt EJ, Laing IA, et al. (1998) The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther 11: 287-290.
- Gill R, Krishnan S, Dozor AJ (2014) Low-level environmental tobacco smoke exposure and inflammatory biomarkers in children with asthma. J Asthma 51: 355-359.
- Spencer N, Blackburn C, Bonas S, Coe C, Dolan A (2005) Parent reported home smoking bans and toddler (18-30 month) smoke exposure: A cross-sectional survey. Arch Dis Child 90: 670-674.
- Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, et al. (2000) Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med 19: 188-192.
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, et al. (1994) The validity of selfreported smoking: a review and meta-analysis. Am J Public Health 84: 1086-1093.
- Nafstad P, Botten G, Hagen JA, Zahlsen K, Nilsen OG, et al. (1995) Comparison of three methods for estimating environmental tobacco smoke exposure among children aged between 12 and 36 months. Int J Epidemiol 24: 88-94.
- Boyaci H, Etiler N, Duman C, Basyigit I, Pala A (2006) Environmental tobacco smoke exposure in school children: parent report and urine cotinine measures. Pediatr Int J 48: 382-389.
- Clark SJ, Warner JO, Dean TP (1994) Passive smoking amongst asthmatic children. Questionnaire or objective assessment? Clin Exp Allergy 24: 276-280.
- Brunekreef B, Leaderer BP, Strien R, Oldenwening M, Smit HA, et al. (2000) PIAMA study group. Using nicotine measurements and parental reports to assess indoor air: The Piama birth cohort study. Epidemiol 11: 350-352.
- Sleiman M, Logue JM, Luo W, Pankow JF, Gundel LA, et al. (2014) Inhalable constituents of third hand tobacco smoke: Chemical characterization and health impact considerations. Environ Sci Technol 48: 13093-13101.
- Olivieri M, Bodini A, Peroni DG, Costella S, Pacifici R, et al. (2006) Passive smoking in asthmatic children: Effect of a “smoke-free house” measured by urinary cotinine levels. Allergy and asthma proceedings. Allergy Asthma Proc 27: 350-353.







