Anton R. Kiselev1*, Vladimir I. Gridnev2 and Olga M. Posnenkova3
1MD, DSc, Leading Researcher, Centre of New Cardiological Informational Technologies, Saratov Research Institute of Cardiology, Saratov, Russia.
2MD, DSc, Head of Centre of New Cardiological Informational Technologies, Saratov Research Institute of Cardiology, Saratov, Russia.
3MD, PhD, Senior Researcher, Centre of New Cardiological Informational Technologies, Saratov Research Institute of Cardiology, Saratov, Russia
- *Corresponding Author:
- Anton R. Kiselev
Centre of New Cardiological Informational Technologies
Saratov Research Institute of Cardiology
141, Chernishevsky str., Saratov, 410028, Russia
E-mail:antonkis@list.ru
Key words
Heart rate variability, chronic heart failure, 0.1 Hz oscillation, load test
Introduction
At present, there are several points of view on the nature of low-frequency (LF) oscillations in heart rate (HR) in humans. According to one hypothesis, these LF oscillations in HR are largely an index of baroreflex gain. [1,2] The existence of a resonance frequency in the 0.1 Hz range in the cardiovascular control is well known. [3-5] Controlled 0.1 Hz breathing maybe used as input stimuli for study of this resonance phenomenon in LF oscillations in HR. Presence of 0.1 Hz resonance phenomenon in HR in chronic heart failure (CHF) patients testifies about the functional status of baroreflex gain and its dysfunction. Implementation of controlled breathing allows a decrease in the influence of additional unregistered factors on the autonomic heart control system, that is impossible during spontaneous breathing. [6,7]
It is especially interesting to study dynamics of LF oscillations in HR during load tests. For example, LF oscillations in HR are used to increase reliability of load test results, for the diagnosis of coronary atherosclerotic stenosis in patients with coronary heart disease. [8] Nevertheless, diagnostic opportunities of load test in CHF patients are not disclosed until now.
Heart rate variability (HRV) is a well-known marker of autonomic dysfunction in CHF patients. [9] However, dynamics of LF oscillations in HR are not investigated in these patients during load tests. The aim of this paper was to study the dynamic characteristics of LF oscillations in HR in CHF patients, during bicycle exercise (BE) tests, under 0.1 Hz controlled breathing.
Methods
Subjects
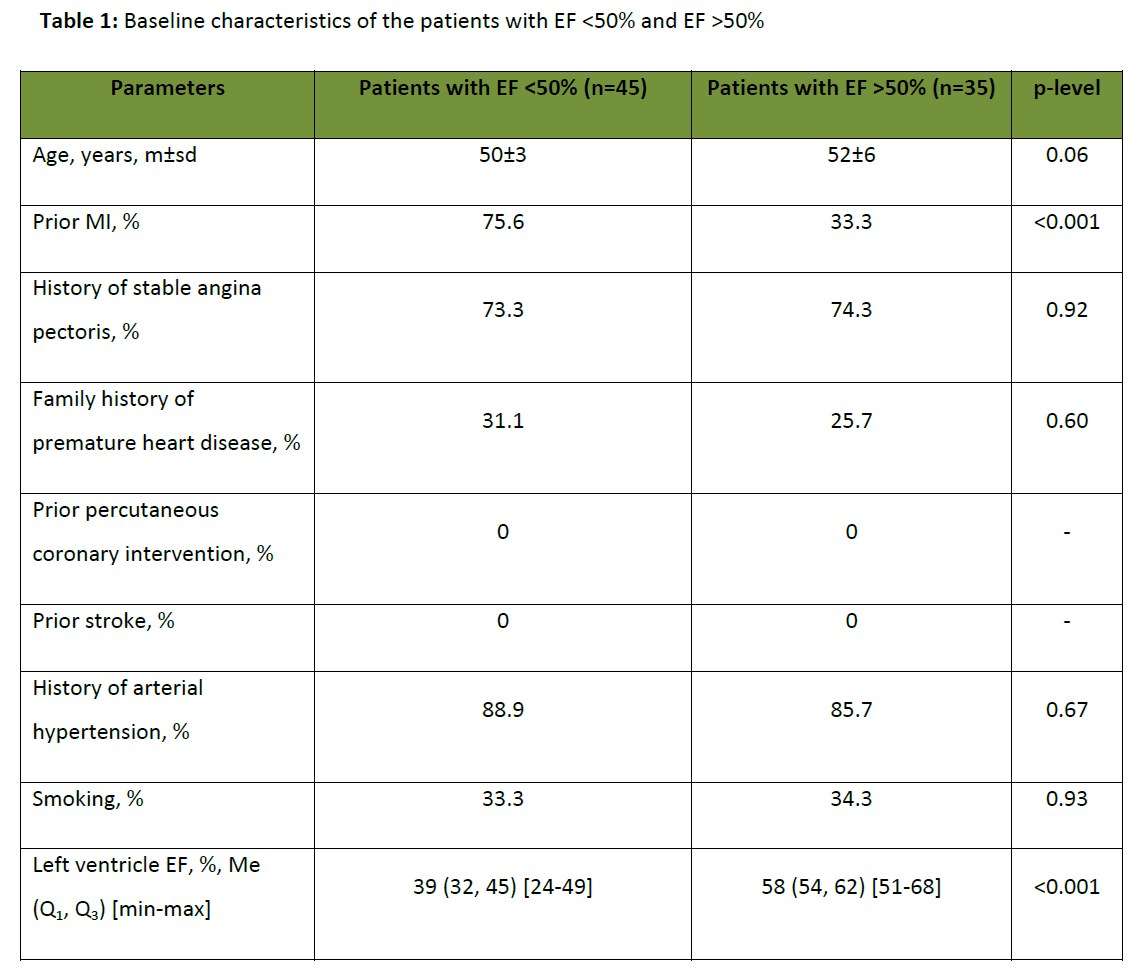
Forty five male CHF patients aged 50±3 years (left ventricle ejection fraction < 50%) and 35 male patients aged 52±6 years without CHF (left ventricle ejection fraction >50%) participated in the study. An informed consent from all volunteers was obtained. The study protocol conformed to the ethical guidelines of 1975 Helsinki Declaration, reflected by a priori approval of the local Ethics Committee.
Baseline characteristics of both groups are shown in table 1. Thirty four patients (75,6%) with ejection fraction (EF) <50% had a history of myocardial infarction (MI), while in the group with normal EF, only 10 patients (33,3%) had a history of MI.
Measurements and protocol
Extracting from the electrocardiography signal a sequence of R-R intervals, i.e., a series of time intervals between the two successive R peaks, we obtained information about the HRV. To obtain equidistant time series from not equidistant sequence of R-R intervals, we approximated it with cubic splines and resample with a frequency of 5 Hz.
A tachogram (R-R interval series) recording was performed in the course of BE tests with loads of 25 W, under 0.1 Hz controlled breathing, for 5 min at each stage of a test. A registration of R-R interval series was performed in sedentary posture and 90 seconds after the beginning of 25 W load stage of BE test. This allowed us to exclude an influence of transients of cardiac function adaptation on the results. The preparation of patients for BE tests included antianginal drugs cessation: for nitrates 24 hours before testing and for beta adrenergic blockers 3-7 days before testing.
The depth and the phase’s ratio of controlled breathing did not differ from those under the spontaneous breathing. The beginning of each inhalation was indicated by the computer-generated sound of duration 0.5 seconds. The subject was asked to inhale when the sound signal appeared. All the subjects were instructed to inhale according to the computer-generated sound and expire spontaneously. No attempts were made to regulate the inhalation/exhalation ratio; the only instruction to the subjects was to maintain their normal breathing depth. There were no other requirements on the character of breathing. Each subject was suggested to choose himself the most comfortable duration of inhale and exhale and the amplitude of breathing. Before starting the protocol all of individuals were trained to breath synchronized with the electronic metronome.
The next day all CHF patients had BE tests with loads of 25 W, under spontaneous breathing. A tachogram (R-R interval series) recording was performed also.
Data analysis
In order to obtain HRV frequency estimates, the parametric method for constructing the spectrum of the temporal R-R series based on the autoregressive model up to the 14th order was used. For further analysis, low-frequency (LF, 0.04–0.15 Hz) bands were used, [9] in which the HRV spectrum frequency power was calculated (in ms2).
R-R interval series free of noise, extra systoles, a noticeable linear trend were selected for spectral analysis.
Statistical analysis
The statistical analysis of the results included the following. To compare the variables between patients’ groups we used the Mann–Whitney test. To compare the variables within one patients’ group we used the Wilcoxon rank test. Continuous variables are reported as medians (Me) with inter-quartile ranges (Q1, Q3) and minimum-maximum range values [min-max] for non-parametric data or mean (M) with standard deviation (sd) for normally distributed data. Categorical data are presented as frequencies and percentages. The obtained estimations were considered statistically significant if p<0.05. For the statistical analysis the software package Statistica 6.1 was used.
Results and discussion
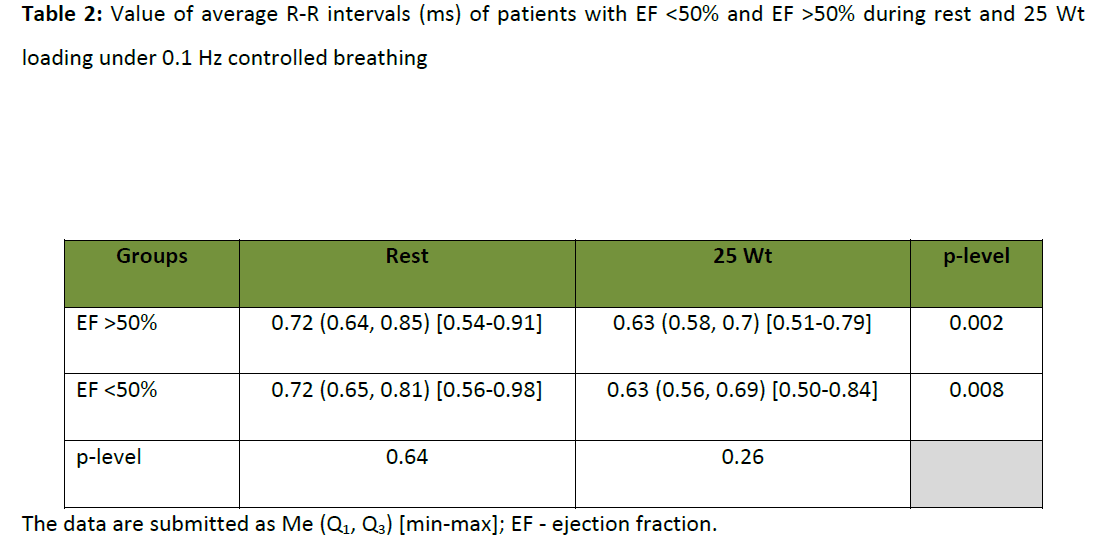
The comparison of average R-R intervals duration (index inverse to HR) under BE test was performed in both of patients’ groups. This is necessary to evaluate the adequacy of study of autonomic influences on HR. It was shown, that in the same conditions of cardiovascular system functioning (relaxed condition and 25 W load) there were no significant differences of average R-R intervals duration in patients with EF<50% and EF>50% (table 2). The balance between sympathetic and parasympathetic departments of the autonomic nervous system in these groups of patients could be interpreted as constant in stipulated conditions of the autonomic heart control system, functioning independently of the severity of a myocardium lesion. In this connection there appeared a necessity of studying of the heart rate variability frequency spectral components, which were independent from prevalence of activity degree of one or the other autonomic nervous system department but characterize a central link of heart rhythm control. In accordance with the concepts of autonomic heart control system functioning listed above, it provoked a direct interest, in particular, to the study of a dynamic of LF component of the HRV spectrum parameters in subjects with different EF.
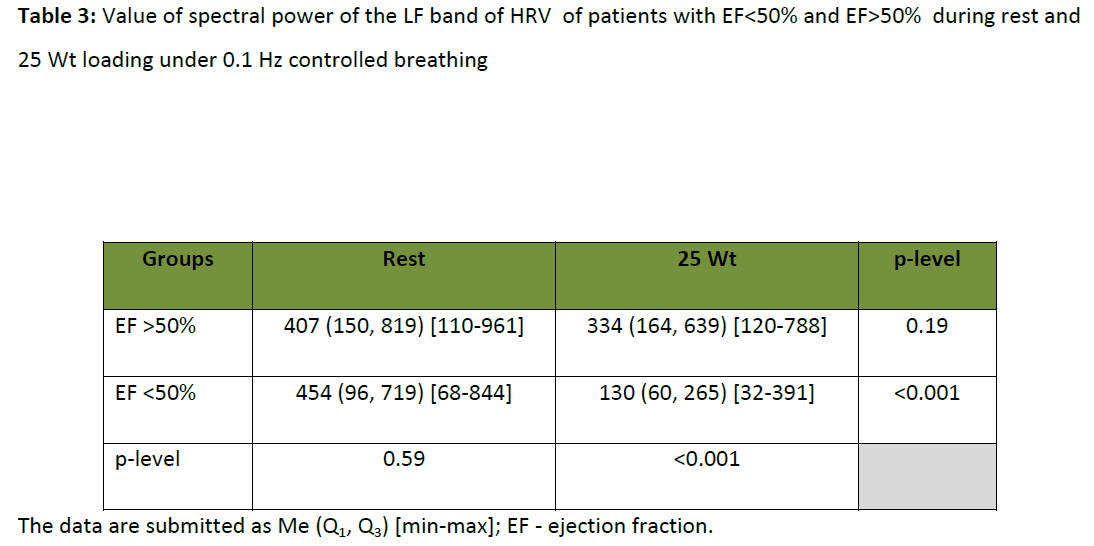
In the process of studying LF band of the HRV spectrum in patients with EF<50% and EF>50% in rest under 0.1 Hz controlled breathing, there were no significant differences in its spectral power (table 3). Consequently, the power of LF band did not display adequately myocardial contractility in the relaxed patients´ condition. It is necessary to take this fact into consideration while interpreting heart rate variability characteristics. But under the load rising till 25 W in patients with myocardial contractility damage, consequent depression of LF component spectral power in 2-3 times in comparison with the relaxed condition was observed under 0.1 Hz controlled breathing (table 3). At the same time, there was no similar reliable expressed dynamic in the group with normal EF. It possible to say that the status of LF oscillations in HR, under 0.1 Hz controlled breathing, in CHF patients with EF>50% is characterized by relative tolerance to low-intensive loads, while in patients with EF<50% these LF oscillations is very unstable to the same loads. Thus, tolerance of LF oscillations in HR to low-intensity loads under 0.1 Hz controlled breathing may be considered to be an independent criterion of dynamic tolerance evaluation of the autonomic heart control in CHF patients.
In most patients low EF was associated with prior MI. Although we have not studied the influence of prior MI on the autonomic heart control in CHF patients. It is known that combination of prior MI and impaired systolic function associated with impaired autonomic heart regulation (estimated by SDNN – standard deviation of beat-to-beats or NN intervals). [10] These three risk-factors determine a greater risk of all-cause and cardiac death within a 5-year period. [10] Also autonomic indexes estimated by HRV have predictive value on long-term outcome in patients with low EF. [11] Perhaps, the study of functional status of baroreflex gain estimated by 0.1 Hz resonance phenomenon in HR will receive more prognostic information in CHF patients with prior MI. However, this hypothesis requires further research.
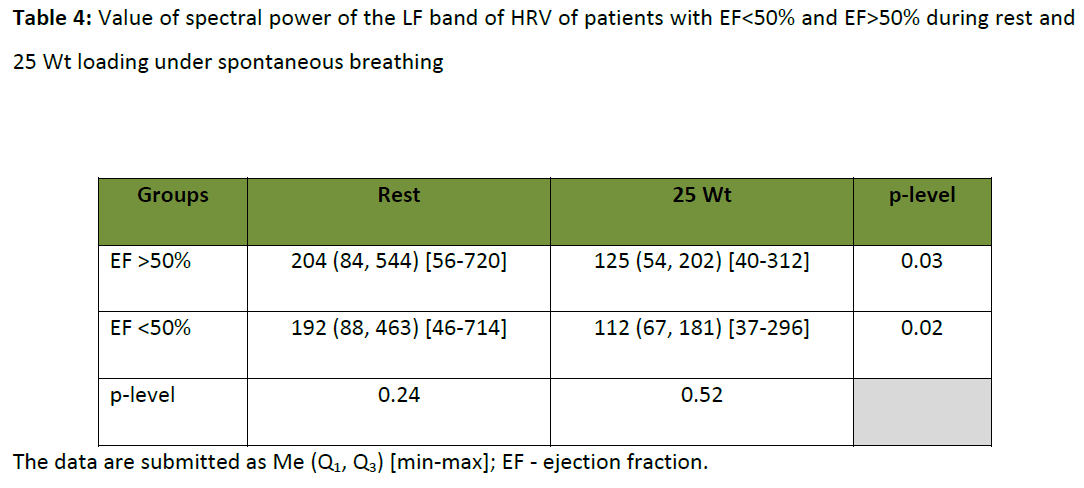
It is also known that the baroreflex sensitivity have clinical significance in CHF patients. [12] Some authors suggest that 0.1 Hz breathing influences on baroreflex sensitivity. [13-15] Y.C. Tzeng et al., [16] concludes that slow breathing does not influence globally the baroreflex sensitivity. We suppose that 0.1 Hz controlled breathing lowers the impact of other factors (internal and external) on LF band of HRV spectrum. This may be due to the resonance phenomenon in LF oscillations in HR. In our study it was shown that there are no differences between the patients with EF<50% and EF>50% in rest and in 25 W load under spontaneous breathing (table 4). Thus, 0.1 Hz controlled breathing is potentially a main external factor for the study of baroreflex gain and its dysfunction in CHF patients.
Conclusions
The stability of LF oscillations in HR to low-intensity loads under 0.1 Hz controlled breathing depends on the severity of myocardial contractility damage. The dynamic of LF band of HRV spectrum may be considered an independent index of dynamic stability of the heart autonomic control in CHD patients
References
- De Boer RW, Karemaker JW, Stracke J. Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. Am J Physiol Heart Circ Physiol. 1987;253: H680–H689.
- Bernardi L, Leuzzi S, Radaelli A, Passino C, Johnston JA, Sleight P. Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: A baroreceptor or central phenomenon? Clin Sci. 1994; 87: 649–654.
- Gridnev VI, Kiselev AR, Kotel’nikova EV, Posnenkova OM, Dovgalevskii PYa, Kirichuk VF. Influence of External Periodic Stimuli on Heart Rate Variability in Healthy Subjects and in Coronary Heart Disease Patients. Human Physiology. 2006; 32(5): 565–573.
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-Frequency Oscillations in Arterial Pressure and Heart Rate; A Simple Computer Model. Am J Physiol (Heart Circ Physiol). 1989; 256: 1573-1579.
- Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, et al. Physiology and Pathophysiology of Heart Rate Variability in Human: Is Power Spectral Analysis Largely an Index of Baroreflex Gain? Clin Sci. 1995; 88: 103-109.
- Radhakrishna KKA, Dutt DN, Yeragani VK. Nonlinear measures of heart rate time series: influence of posture and controlled breathing. Autonomic Neuroscience - Basic & Clinical. 2000; 83(3): 148-158.
- Patwardhan A, Evans J, Bruce E, Knapp C. Heart rate variability during sympatho-excitatory challenges: comparison between spontaneous and metronomic breathing. Integr Physiol Behav Sci. 2001; 36(2): 109-120.
- Gridnev VI, Kiselev AR, Posnenkova OM, Shvartz VA. Using of spectral analysis of heart rate variability for increasing reliability of bicycle ergometry results. Health. 2011; 3(8): 477-481.
- Heart Rate Variability. Standards of Measurement, Physiological Interpretation and Clinical Use. Circulation. 1996; 93: 1043-1065.
- Ablonskytė-Dūdonienė R, Bakšytė G, Ceponienė I, Kriščiukaitis A, Drėgūnas K, Ereminienė E. Impedance cardiography and heart rate variability for long-term cardiovascular outcome prediction after myocardial infarction. Medicina (Kaunas). 2012; 48(7): 350-358.
- La Rovere MT, Pinna GD, Maestri R, Barlera S, Bernardinangeli M, Veniani M, et al. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI-HF trial. Eur J Heart Fail. 2012 Jul 30. [Epub ahead of print] doi: 10.1093/eurjhf/hfs126.
- Nepoklonov MV, Rogoza AN, Skvortsov AA, Gorieva ShB, Tereshchenko SN. Baroreflex sensitivity in patients with chronic heart failure: the clinical significance and impact of therapy. Ter Arkh. 2010; 82(12): 73-80. [Article in Russian]
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002; 105: 143-145.
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005; 46: 714–718.
- Radaelli A, Raco R, Perfetti P, Viola A, Azzellino A, Signorini MG, Ferrari AU. Effects of slow, controlled breathing on baroreceptor control of heart rate and blood pressure in healthy men. J Hypertens. 2004; 22: 1361–1370.
- Tzeng YC, Sin PY, Lucas SJ, Ainslie PN. Respiratory modulation of cardiovagal baroreflex sensitivity. J Appl Physiol. 2009; 107(3): 718-724.
3063
References
- De Boer RW, Karemaker JW, Stracke J. Hemodynamic fluctuations and baroreflex sensitivity in humans: A beat-to-beat model. Am J Physiol Heart Circ Physiol. 1987;253: H680–H689.
- Bernardi L, Leuzzi S, Radaelli A, Passino C, Johnston JA, Sleight P. Low-frequency spontaneous fluctuations of R-R interval and blood pressure in conscious humans: A baroreceptor or central phenomenon? Clin Sci. 1994; 87: 649–654.
- Gridnev VI, Kiselev AR, Kotel’nikova EV, Posnenkova OM, Dovgalevskii PYa, Kirichuk VF. Influence of External Periodic Stimuli on Heart Rate Variability in Healthy Subjects and in Coronary Heart Disease Patients. Human Physiology. 2006; 32(5): 565–573.
- Madwed JB, Albrecht P, Mark RG, Cohen RJ. Low-Frequency Oscillations in Arterial Pressure and Heart Rate; A Simple Computer Model. Am J Physiol (Heart Circ Physiol). 1989; 256: 1573-1579.
- Sleight P, La Rovere MT, Mortara A, Pinna G, Maestri R, Leuzzi S, et al. Physiology and Pathophysiology of Heart Rate Variability in Human: Is Power Spectral Analysis Largely an Index of Baroreflex Gain? Clin Sci. 1995; 88: 103-109.
- Radhakrishna KKA, Dutt DN, Yeragani VK. Nonlinear measures of heart rate time series: influence of posture and controlled breathing. Autonomic Neuroscience - Basic & Clinical. 2000; 83(3): 148-158.
- Patwardhan A, Evans J, Bruce E, Knapp C. Heart rate variability during sympatho-excitatory challenges: comparison between spontaneous and metronomic breathing. Integr Physiol Behav Sci. 2001; 36(2): 109-120.
- Gridnev VI, Kiselev AR, Posnenkova OM, Shvartz VA. Using of spectral analysis of heart rate variability for increasing reliability of bicycle ergometry results. Health. 2011; 3(8): 477-481.
- Heart Rate Variability. Standards of Measurement, Physiological Interpretation and Clinical Use. Circulation. 1996; 93: 1043-1065.
- Ablonskytė-Dūdonienė R, Bakšytė G, Ceponienė I, Kriščiukaitis A, Drėgūnas K, Ereminienė E. Impedance cardiography and heart rate variability for long-term cardiovascular outcome prediction after myocardial infarction. Medicina (Kaunas). 2012; 48(7): 350-358.
- La Rovere MT, Pinna GD, Maestri R, Barlera S, Bernardinangeli M, Veniani M, et al. Autonomic markers and cardiovascular and arrhythmic events in heart failure patients: still a place in prognostication? Data from the GISSI-HF trial. Eur J Heart Fail. 2012 Jul 30. [Epub ahead of print] doi: 10.1093/eurjhf/hfs126.
- Nepoklonov MV, Rogoza AN, Skvortsov AA, Gorieva ShB, Tereshchenko SN. Baroreflex sensitivity in patients with chronic heart failure: the clinical significance and impact of therapy. Ter Arkh. 2010; 82(12): 73-80. [Article in Russian]
- Bernardi L, Porta C, Spicuzza L, Bellwon J, Spadacini G, Frey AW, et al. Slow breathing increases arterial baroreflex sensitivity in patients with chronic heart failure. Circulation. 2002; 105: 143-145.
- Joseph CN, Porta C, Casucci G, Casiraghi N, Maffeis M, Rossi M, Bernardi L. Slow breathing improves arterial baroreflex sensitivity and decreases blood pressure in essential hypertension. Hypertension. 2005; 46: 714–718.
- Radaelli A, Raco R, Perfetti P, Viola A, Azzellino A, Signorini MG, Ferrari AU. Effects of slow, controlled breathing on baroreceptor control of heart rate and blood pressure in healthy men. J Hypertens. 2004; 22: 1361–1370.
- Tzeng YC, Sin PY, Lucas SJ, Ainslie PN. Respiratory modulation of cardiovagal baroreflex sensitivity. J Appl Physiol. 2009; 107(3): 718-724.









