Background
The incidence of portal vein thrombosis (PVT) after liver transplantation (LT) is considered to be relatively an uncommon complication (1-3%) in comparison to hepatic artery thrombosis, however it can significantly reduce graft and patient survival. Delayed PVT, defined as those appeared one month after LT, does not necessarily lead to graft failure and the main consequences are related to portal hypertension. To the contrary, early PVT potentially resulted in re-transplantation [1-3].
Virchow describes three broad categories of factors that are thought to contribute to thrombosis: hypercoagulability, hemodynamic changes and endothelial injury. In low pressure systems (as it is venous circulation) the hemodynamic factor (blood flow) is considered to play an important role in the development of thrombotic events. For this reason, intraoperative flow measurements (arterial and portal flows) are performed in majority of centers in all patients before bile duct anastomosis during LT. However, these flows are subjected to hemodynamic patient conditions (especially cardiac index and vascular resistances) which are highly variable during and immediately after liver transplantation [4]. Otherwise, some groups suggest the importance of the temporary porto-caval shunt performed during the anhepatic phase to know the conditions before reperfusion and determine whether they will be favorable or not [5].
Although as to be one of the main factors, the role of intraoperative portal flow after graft reperfusion as a contributor to PVT after liver transplantation has not be assessed yet. Our aim is to investigate the correlation between the intraoperative portal flows after graft reperfusion with the appearance of early portal vein thrombosis after LT. Secondarily; we investigate the correlation between the temporary porto-caval shunt and the portal flow, also between the cardiac output and the temporary porto-caval shunt and arterial and portal flows after graft reperfusion, during LT.
Material and Methods
We retrospectively evaluated 452 consecutive LT performed at the Hospital Clinic of Barcelona between January 2009 and May 2015. We excluded 24 liver-kidney, 2 liver-heart combined transplants, 3 patients with Budd Chiari syndrome, and patients who undergo early re-transplant (less than 30 days after the first one) due to primary non function or arterial thrombosis (n = 7). Finally, 416 patients were included in the study. The data were collected prospectively and were analyzed retrospectively. Preoperative thrombophilia screening was routinely done in those patients with PVT prior to LT. The MELD (model for end-stage liver disease) score was weighted for hepatocarcinoma according to Sharma and col (range, 6-40) [6]. General anesthesia was performed as previously described [7], and blood products were transfused according to our protocol started from 2007 [8].
Hepatectomy was performed with systematic preservation of the inferior vena cava as well as a temporary terminolateral porto-caval shunt fashioned with continuous 5-0 polypropylene suture. Graft was flushed with 500 cc of Lactate Ringer at 37 C immediately before portal reperfusion. Porto-caval shunt was routinely sectioned before graft implantation, to perform the anastomosis between the portal vein of the recipient and the portal vein of the graft with continuous 5-0 polypropylene suture. Finally, the hepatic artery anastomosis (with continuous 6-0) and the bile duct anastomosis were completed.
Intraoperative thrombectomy was performed in case of PVT prior to LT, when needed. As it is routine at our institution, intraoperative hepatic artery and portal vein flow in all patients were measured before bile duct anastomosis. Temporary portocaval shunt flow was also measured during the anhepatic phase. After the measurement of the portal and hepatic artery flows, the portal vein was clamped to assess the augmentation of the hepatic artery flow. Splenic artery ligation was performed when needed, and flow was reassessed after it. Measurements were made with the method for determining the transit time flow, with 8-mm to 12-mm probes used for the portal vein and 3-mm to 5-mm probes used for the hepatic artery; they were monitored with the VeriQ 1001 system (Medi-Stim ASA, Oslo, Norway). Thromboprophylaxis was not routinely given after LT unless intraoperative thrombectomy was performed. If postoperative portal vein thrombosis occurred, anticoagulation was achieved by using low molecular weight heparin or heparin infusion targeting the PTTA values below 1, 5 the upper normal limit. Immunosuppression was administered based on the protocols established at our center. PVT was defined as the absence of flow in part or all the lumen of the portal vein trunk, or portal vein branches, with the presence of solid material within the vein, as documented by doppler ultrasound, and/or angiography; PVT was defined as early or late according to its appearance was before or after 30 days post LT. Medical records were reviewed until 6 months after surgery, death, or re-transplantation. The ethics and research committee of the Hospital Clinic of Barcelona has approved the study.
Statistical methods
Continuous variables were expressed as median and interquartile range. The chi-square test or the Fisher’s exact tests were used for qualitative or dichotomized variables, and the Mann-Whitney test for continuous variables. A two-tailed p value of less than 0.05 was taken as representing significance. Logistic regression analysis was used to find predictive factors for post-operative venous thrombotic event. All analyses were performed with computer software (SPSS, Version 18, SPSS, Inc., Chicago, IL).
Results
Baseline data characteristics of the 416 LTs at the time of surgery are summarized in Table 1. Seventy-one percent of patients were men, with a median age of 56 (50-62) years, MELD 18 (11-23); hepatitis C virus (HCV) positive was the most prevalent etiology (47%).
| |
All |
PVT after LT yes |
PVT after LT no |
p |
| n =416 |
n =5 |
n =411 |
| Age, y |
56(49-62) |
60(51-62) |
56(49-62) |
0.55 |
| Sex, m(%) |
295(71) |
1(20) |
291(71) |
0.9 |
| Body surface, m2 |
1.8(1.7-1.9) |
1.7(1.6-1.9) |
1.8(1.7-1.9) |
0.35 |
| MELD |
18(11-23) |
25(18-27) |
18(11-23) |
0.11 |
| Child |
9(6-11) |
11(9-12) |
9(6-11) |
0.38 |
| PVT pre LT |
42(10) |
5(100) |
37(9) |
<0.01 |
| Etiology of liver disease, n(%) |
|
|
|
0.14 |
| |
|
|
|
| HCV |
196(47) |
1(60) |
195(46) |
| OH |
88(21) |
3(20) |
85(20) |
| AFL |
27(7) |
|
27(8) |
| Biliary cirrhosis |
22(5) |
|
22(6) |
| HBV |
19(4) |
|
19(4) |
| FAP |
13(3) |
|
13(3) |
| Crriptogenic |
10(3) |
|
10(3) |
| Autoimmune |
7(2) |
1(20) |
6(2) |
| Others |
34(8) |
|
34(8) |
| HCC, n(%) |
174(42) |
1(20) |
173(42) |
0.65 |
| Type of donor, n(%) |
|
|
|
0.64 |
| DBD |
354(85) |
4(80) |
350(84) |
| LD |
29(7) |
1(20) |
28(8) |
| DCD |
25(6) |
|
25(6) |
| FAP |
8(2) |
|
8(2) |
| Intraoperative transfusion |
|
|
|
|
| RBC, units |
2(0-4) |
4(0-5) |
2(0-4) |
0.58 |
| FFP, ml |
0(0-982) |
491(0-646) |
0(0-991) |
0.86 |
| Platelets, n(%) |
69(17) |
1(20) |
68(17) |
0.95 |
| Fibrinogen, n(%) |
175(42) |
2(40) |
173(42) |
1 |
| Tranexamic acid, y(%) |
200(48) |
3(60) |
197(48) |
0.67 |
| Surgical time, min |
330(295-385) |
285(250-412) |
330(295-385) |
0.17 |
| Cold isquemia, min |
391(300-495) |
430(250-495) |
390(300-495) |
0.98 |
| Warm ischemia, min |
30(25-40) |
35(25-45) |
30(25-40) |
0.82 |
| PVT: Portal Vein Trombosis;LT: Liver Transplantation; HCV: Hepatitis C Virus; OH: Alcoholic; ALF: Acute Liver Failure; HBV: Hepatitis B Virus; FAP: Familial Amyloidotic Polyneuropathy; Others: Alpha 1 Antitrypsin Deficiency, Congenital Hepatic Fibrosis, and Wilson Disease; DBD: Donor After Brain Death; LD: Living Donors; DCD: Donor After Cardiac Death; RBC: Red Blood Cells; FFP: Fresh Frozen Plasma. Values Given as a Median (Interquartile Range). |
Table 1: Demographic and intraoperative data of the 416 LT.
During the anhepatic phase, values of the temporary porto-caval shunt were of 1350 ± 644 (range from 1000-1750) ml/min. These values correspond to final measurements. In a total of 4 cases porto-caval shunt blood flow was extremely low (<700 ml/min). Identification and ligation of the spontaneous porto-systemic shunt allowed to obtain an adequate blood flow (>1000 ml/min).
At the time of reperfusion, arterial and portal vein blood flows were 220 ± 240 (160-300) ml/min and 1500 ± 666 (1200-2000) ml/ min, respectively. There were any significant differences in values between patients who experienced postoperative PVT vs. those who did not (Table 2). The overall incidence of postoperative thrombosis was (5/416, 1,2%). On the other hand, the incidence of preoperative PVT was 10% (42 patients). This fact proves to be a significant factor at the time of developing postoperative PVT: all patients (5 patients) that developed a postoperative PVT, had a partial or complete preoperative PVT.
| |
All |
PVT after LT yes |
PVT after LT no |
P |
| N=416 |
n =5 |
N =411 |
| Cl |
3.9(3.2-5.1) |
3.4(3.2-4.5) |
3.9(3.2-5.1) |
0.4 |
| Portal flow |
1500(1200-2000) |
1500(1150-2300) |
1500(1200-2000) |
0.9 |
| Portal flow/Cl |
842(641-1147) |
797(627-1376) |
843(641-1147) |
0.9 |
| Arterial flow |
220(160-300) |
180(141-525) |
220(160-300) |
1 |
| Arterial flow/Cl |
120(88-162) |
93(77-312) |
120(87-161) |
1 |
| PC shunt flow |
1350(1000-1750) |
1500(800-) |
1350(1000-1750) |
0.8 |
| PC shunt flow/Cl |
755(558-948) |
781(462-) |
754(559-946) |
1 |
| PVT: Portal Vein Thrombosis;Cl: Cardiac Index; LT: Liver Transplantation. |
Table 2: Intraoperative portal, arterial and porto-caval shunt flow.
Thrombophilic study was positive in 8 of the 42 patients with PVT prior to LT (19%) (Table 1). No patients with positive thrombophilic study experienced PVT after LT. Data of patients with PVT after LT is shown in (Table 3). Re-intervention was required in one patient 24h after LT and the rest were successfully managed with anticoagulation by heparin perfusion initially, and low molecular weight heparin thereafter. After six months, all patients maintained a within normal liver function.
| |
PVT pre |
Days after LT |
CO |
CL |
Portal flow |
Portal flow BSA |
Arterial flow |
Arterial flow BSA |
Thrombophilic study* |
| LT |
| 1 |
yes |
1 |
1.8 |
3 |
1500 |
797 |
170 |
90 |
negative |
| 2 |
yes |
1 |
2 |
3 |
1000 |
578 |
113 |
65 |
negative |
| 3 |
yes |
1 |
1.6 |
3 |
1300 |
677 |
180 |
93 |
negative |
| 4 |
yes |
7 |
3.1 |
6 |
3000 |
1714 |
740 |
442 |
negative |
| 5 |
yes |
28 |
2.3 |
4 |
1600 |
1038 |
310 |
201 |
negative |
| LT: Liver Transplantation; CO: Cardiac Output (L/min); CL: Cardiac Index (L/min/m2), BSA: Body Surface Area (m2); *Done before LT. |
Table 3: Data of 5 patients with PVT after LT.
Mean values of temporary porto-caval shunt during the anhepatic phase correlated significantly with the portal vein flow after reperfusion of the liver graft (R2 0.2, P < 0.001) (Figure 1). After graft reperfusion, cardiac output moderately correlated with portal flow (R2 0.1, P < 0.001); but it did not correlate with temporary porto-caval shunt flow, neither with the hepatic artery flow (R2 0.05, P < 0.001), (R2 3e-4, P ns) respectively (Figure 2).
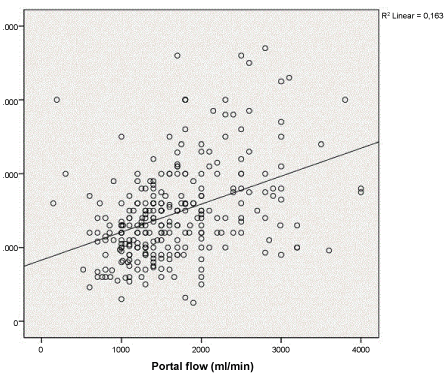
Figure 1: Correlations between porto-caval shunt flow and portal flow.

Figure 2: Correlations between cardiac output and porto-caval shunt flow, arterial flow and portal flow. CO: Cardiac Output; BSA: Body Surface Area.
Discussion
The incidence of PVT after LT in the present series was 1.5%; after adjusting for cardiac output and body surface area, there were no differences in the intraoperative portal flow after graft reperfusion between patients who experienced postoperative PVT and those who did not. These results confirm that PVT after LT is a relatively infrequent event, and suggest that intraoperative portal flow may not be useful to detect patients at high risk of PVT.
Taking into account the importance of Virchow’s triad in the pathogenesis of vascular thrombosis, poorly attention is taken until now to the intraoperative blood flow as a contributor to PVT [9]. In our knowledge, this is the first study that aims to correlates the intraoperative portal flow after graft reperfusion with the occurrence of postoperative PVT.
Hepatic and systemic hemodynamic are significantly altered during LT mainly due to general anesthesia, the use of vasopressor drugs and sudden shift of fluids as well [10]. There is an increase in portal flow and a decrease in mean arterial pressure after reperfusion that usually recovers progressively; however, total recovery to normal hemodynamic values may take 2 weeks after LT, with marked reduction in cardiac index and increases in mean arterial pressure, also peripheral vascular resistance [11]. Even two months after LT, further significant increases in peripheral vascular resistance were observed. Since portal flow is highly dependent of cardiac output, it is not surprising that intraoperative measurements of the portal blood flow can hardly predict portal flow abnormalities that can potentially be involved in postoperative PVT. The poor impact of the portal flow on the occurrence of the PVT in this series, contrast with the contribution to the portal flow described on the development of PVT prior to LT in cirrhotic population, in which decreased portal flow (specifically, portal flow velocity below 15 cm/sc) is a recognized risk factor for this complication [12].
On the other hand, PVT before LT was the only different variable between patients with and without PVT after LT: All PVT after LT were actually, re-thrombosis. In patients with portal vein thrombosis at the time of transplantation, whit the objective to optimize portal blood flow to the graft, the removal of the clot or the clot and the attached intimal layer of the vein is frequently needed (thrombectomy or thrombendvenectomy) [13-16]. This maneuver can injure the endothelial in the thrombus side, making them more prone to re-thrombosis. Considering that the thrombophilic study, the third main contributor to thrombosis, was negative in all LT recipients who displayed PVT after LT, local factors may have a determinant role promoting PVT in this population. In fact, although controversial, re-thrombosis is a complication more frequently reported in patients with pre transplant PVT, despite properly restoration of the blood flow [1].
We found a moderate correlation between portal flow and temporary porto-caval shunt flow. This result was in accordance with previous results reported by our group in partial grafts. Even though in whole graft this information is not so relevant [17], this finding could be used to identify patients at risk of insufficient portal flow after graft reperfusion, pointing out the need to seek a porto-systemic shunt, which after to be occluded, could improves the portal flow. In our institution, systematic measurements at temporary porto-caval shunt completion, allowed us to identify those patients with a spontaneous porto-systemic shunt and a compromised portal blood flow. Identification of the shunt either preoperatively or during the procedure was of paramount importance in order to obtain a significant flow at reperfusion [5]. For this reason the range of the portal flows was always within adequate limits.
Cardiac index was positively correlated whit portal blood, whereas it was not correlated with hepatic artery flow; it is well know that portal flow is mainly dependent on cardiac output, whereas hepatic artery is able to self-regulate the flow [18].
This study shows several weaknesses, in addition to this retrospective nature, the sample size is too small considering the low prevalence of the inquired event, preventing definitive conclusions; all this limitation factors that may do not permit to dress a conclusion; however, it is strongly suggested that intraoperative portal flow is not a good parameter to identify patients at risk of PVT after LT. Moreover, in addition to blood flow, the resistive index which is the ratio of (peak systolic velocity− peak diastolic velocity)/peak systolic velocity) considered as more accurate predictors, was not recorded. Finally, portal flow can be influenced by the graft quality, which was not assessed (e.g. after reperfusion liver biopsy).
To sum up, the results from the present study reassert that early PVT is an infrequent complication after LT, and point to local factors as contributors of this development; whereas portal flow measured after graft reperfusion is not appeared to be able to predict PVT. Even though, porto-caval temporary shunt flow could be used to identify those patients who need some vascular intervention in order to improve the portal flow after reperfusion.
9047
References
- Duffy JP, Hong JC, Farmer DG, Ghobrial RM, Yersiz H, et al. (2009) Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am CollSurg 208: 896-903.
- Ponziani FR, Zocco MA, Senzolo M, Pompili M, Gasbarrini A, et al. (2014) Portal vein thrombosis and liver transplantation: implications for waiting list period, surgical approach, early and late follow-up. Transplant Rev (Orlando) 28: 92-101.
- Qi X, Dai J, Jia J (2015) Association between Portal Vein Thrombosis and Survival of Liver Transplant Recipients: a Systematic Review and Meta-analysis of Observational Studies. J Gastrointestin Liver Dis24:51-59.
- Gu L-H, Fang H, Li F-H (2015) Preoperative hepatic hemodynamics in the prediction of early portal vein thrombosis after liver transplantation in pediatric patients with biliary atresia. HepatobiliaryPancreat Dis Int14:380-385.
- Sánchez-Cabús S, Abraldes JG, Taurá P, Calatayud D, Fondevila C, et al. (2014) Lack of correlation between preoperative and intraoperative liver hemodynamics: a descriptive analysis. Transplantation 97: 116-121.
- Wiesner R, Edwards E, Freeman R, Harper A, Kim R, et al. (2003) Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology 124: 91-96.
- Escobar B, Taurá P, Martínez-Palli G, Fondevila C, Balust J, et al. (2014) Stroke volume response to liver graft reperfusion stress in cirrhotic patients. World J Surg 38: 927-935.
- Blasi A, Beltran J, Pereira A, Martinez-Palli G, Torrents A, et al. (2012) An assessment of thromboelastometry to monitor blood coagulation and guide transfusion support in liver transplantation. Transfusion 52: 1989-1998.
- Kinjo N, Kawanaka H, Akahoshi T, Matsumoto Y, Kamori M, et al. (2014) Portal vein thrombosis in liver cirrhosis. World J Hepatol 6: 64-71.
- Paugam-Burtz C, Kavafyan J, Merckx P, Dahmani S, Sommacale D, et al. (2009) Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl 15: 522-529.
- Navasa M, Feu F, García-Pagán JC, Jiménez W, Llach J, et al. (1993) Hemodynamic and humoral changes after liver transplantation in patients with cirrhosis. Hepatology 17: 355-360.
- Zocco MA, Di Stasio E, De Cristofaro R, Novi M, Ainora ME, et al. (2009) Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 51: 682-689.
- Wu TH, Lin YS, Lee CF, Wu TJ, Yu MC, et al. (2011) Clinical analysis and strategy for liver transplantation in patients with pre-existing portal vein thrombosis. Chang Gung Med J 34: 426-434.
- Gao PJ, Gao J, Li Z, Hu ZP, Leng XS, et al. (2015) Liver transplantation in adults with portal vein thrombosis: Data from the China Liver Transplant Registry.Clin Res HepatolGastroenterol.
- Ramos AP, Reigada CP, Ataíde EC, Almeida JR, Cardoso AR, et al. (2010) Portal vein thrombosis and liver transplantation: long term. Transplant Proc 42: 498-501.
- Robles R, Fernandez JA, Hernández Q, Marín C, Ramírez P, et al. (2004) Eversion thromboendovenectomy in organized portal vein thrombosis during liver transplantation. Clin Transplant 18: 79-84.
- Hessheimer AJ, Escobar B, Muñoz J, Flores E, Gracia-Sancho J, et al. (2014) Somatostatin therapy protects porcine livers in small-for-size liver transplantation. Am J Transplant 14: 1806-1816.
- Dalal A (2016) Anesthesia for liver transplantation. Transplant Rev (Orlan do) 30: 51-60.