Ketan Soni*and Kavita Sharma
Department of Forensic Science, University of Shri Vaishnav Vidyapeeth Vishvavidhyalaya, Gram Baroli, Indore, Madhya Pradesh, India
*Corresponding Author:
Ketan Soni
Department of Forensic Science
University of Shri Vaishnav Vidyapeeth Vishvavidhyalaya
Gram Baroli
Indore
Madhya Pradesh
India
E-mail: ketan.soni5050@gmail.com
Received Date: March 03, 2021 Accepted Date: March 17, 2021 Published Date: March 24, 2021
Citation: Soni K, Sharma K (2021) Eco-Friendly Spectrophotometric Analysis of the Poorly Water-Soluble Drug (Satranidazole) using the Mixed Hydrotropic Concept. Int J Drug Dev & Res Vol.13 No.2: 155.
Copyright: © 2021 Soni K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Spectrophotometric analysis; Hydrotropic theory; Satranidazole
Introduction
Hydrotropy is one of the best procedures accessible to solving problems of solubility in these technologies. One of the crucial complicated issues of maximum drugs is low solubility [1-3]. Several organic solvents are utilized in the estimation of drugs. High cost, volatility, and toxicity are the disadvantages of these organic solvents. If swallowed, breathed in, or ingested through the skin, organic solvents are destructive. These solvents are seldom utilized in the category of Class 2 solvents, i.e. solvents, according to I.C.H guideline Q3 CR3 (impurity guideline for residual solvents). There is therefore a fundamental need for spectrophotometric analysis to substitute organic solvent with a protected, eco-friendly, cost-effective solvent [4,5]. In a declaration to boost the aqueous solubility of a huge number of low water-soluble drugs, concentrated aqueous solutions of sodium benzoate, sodium acetate, urea, niacinamide, sodium caprylate, and sodium citrate were assigned [6]. In the mixed hydrotropic process, two or more hydrotropic agents are utilized, giving the purpose of decreased toxicity and single hydrotropic concentration. One of the best methods to increase the water solubility of poorly water-soluble drugs is the mixed hydrotropic theory [7-9]. To ignore the usage of organic solvents, the mixed hydrotropic concept could be an acceptable alternative. Thus in quantitative analysis of low water-soluble drugs, there is a wide scope for mixed hydrotropic principles [10-13]. The latest research work offers an eco-friendly path of estimating the satranidazole drug spectrophotometrically in tablet formulations without the support of organic solvents.
Materials and Methods
Satranidazole was collected from the laboratory of Alkem Ltd. Mumbai and satranidazole tablets were obtained from Indore's local market from two separate firms (Alkem Laboratory Ltd. and Indchemie Health Specialties Pvt Ltd.). Analytical grade chemicals were utilized.
Instrumentation
For spectrophotometric estimation, the UV Visible spectrophotometer (Model 1800, Shimadzu) was utilized.
Investigations of preliminary solubility
Distilled water and mixed solvent blend (containing 25% Sodium benzoate and 30% phenol) were transferred in a 25 ml bottle and add sufficient amount of drug in it, shake the bottle for 12 hours (at room temperature) with an orbital flask shaker and keep the bottle for 24 hours so that its solution remains undisturbed and then with the help of what man filter paper. Filtered the solution and diluted the filtrate with distilled water and noted its absorbance at 390 nm against reagent blanks.
The preparation of the drug's calibration curve (satranidazole)
50 mg of the standard drug satranidazole and 8 ml of the blend were accurately weighed and transferred to a 10 ml volumetric flask. After precise dissolution, complete dissolution of the drug was performed by shaking the flask, to make up the volume up to 10 ml enough blend was added. Different standard solutions of 10, 20, 30, and 40 μg/ml concentrations were prepared from this stock solution by suitable dilution with distilled water. At 390 nm against the respective reagent blank, the absorbances of these solutions were noted (Table 1 and Figure 1).
| S.no. |
Concentration (µg/ml) |
Absorbance |
| 1 |
0 |
0 |
| 2 |
10 |
0.36 |
| 3 |
20 |
0.678 |
| 4 |
30 |
1.083 |
| 5 |
40 |
1.44 |
Table 1: Data of calibration curve.
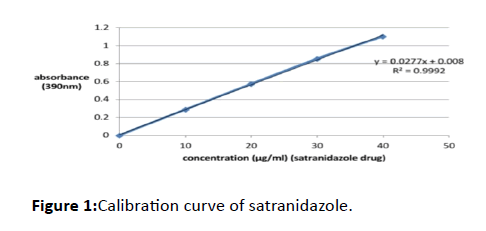
Figure 1: Calibration curve of satranidazole.
Proposed research approach
In a 10 milliliter volumetric flask, tablet powder (equal to 50 milligrams of satranidazole drug) and 8 ml of blend solution was shifted. The flask was energetically shaken for 15 minutes, and an applicable blend solution was pertained to make up to 10 milliliters of volume. Filtration was done (via Whatman filter paper no. 41) for the elimination of the tablet excipients. Dilution of the 0.6 ml filtrate by d/w to 100 ml and absorbance against the reagent blank was noted at 390 nm (Table 2).
| Tablets of satranidazole drug |
Amount of drug showed(mg/tablet) |
% drug calculated (mean ± Standard deviation ) |
% coefficient of variation |
Standard error |
| I (Alkem Laboratory Ltd.) |
300 |
102.69 ± 0.959 |
0.933 |
0.538 |
| II (Indchemie Health Ltd.) |
300 |
101.04 ± 0.835 |
0.826 |
0.476 |
Table 2: Proposed research approach.
Recovery studies
The regular satranidazole drug (20 mg and 40 mg separately) was added to the pre-analyzed tablet powder equal to 50 mg satranidazole to conform with the recovery investigations and the drug quantity was calculated by the proposed procedure. Both forms of analysis have been replicated three times. In Table 3, the results of the study are listed.
| Tablets of satranidazole drug |
Drug quantity in pre-analyzed tablet powder (mg) |
Quantity of standard drug added (mg) (spiked) |
% drug calculated (mean ± Standard deviation ) |
% coefficient of variation |
Standard error |
| I (Alkem) |
50 |
20 |
100.53 ± 0.829 |
0.824 |
0.475 |
| I (Alkem) |
50 |
40 |
98.12 ± 0.813 |
0.828 |
0.249 |
| II (Indchemie) |
50 |
20 |
99.73 ± 0.749 |
0.751 |
0.478 |
| II (Indchemie) |
50 |
40 |
98.2 ± 0.212 |
0.216 |
0.124 |
Table 3: Outcomes of recovery studies using statistical investigation (n=3).
Results and Discussion
It is established that the solubility of satranidazole in distilled water (at room temperature) was 0.641 mg/ml, and 190 mg/ml in the blend solution. Table 2 shows the value of percent estimation obtained from 101.04 (Table 3) to 102.69 (Table 2). This value is obtained by spectrophotometric analysis with the help of the mixed hydrotropic technique. This value is obtained near 100 so we can say that the proposed method is correct. Standard deviation (0.835 to 0.959), percentage coefficient of variation (0.826 to 0.933) and the value of standard error (0.476 to 0.538) are also very low hence we can say that the proposed method is accurate.
Table 2 shows the value of the recovery studies that have been achieved from 98.12 to 100.53. This value is also close to 100 which shows the precision of the proposed method, as well as the standard deviation (0.212 to 0.829), percentage coefficient of variance (0.216 to 0.828), and the standard error (0.124 to 0.478) value also very low, which shows the validation of the proposed method.
Currently, due to their special characteristics such as fast availability, good recovery, absence of fire risks, and eco-friendly design, hydrotropic solutions have strong industrial needs. In the pharmaceutical region, a mixed hydrotropic technique can be assigned efficiently. It can be adopted to estimate spectrophotometrically low water-soluble drugs from the bulk drug sample to avoid the usage of organic solvents that provide quick, inexpensive, environmentally friendly, safe, and reliable analytical mechanisms.
Conclusion
It may be inferred that it is possible to use the Mixed Hydrotropic Approach to replace the use of an organic solvent that is more expensive and harmful for our atmosphere. For the spectrophotometric study of other poorly water-soluble drugs avoiding the use of organic solvents, there is a further scope of a hydrotropic mixture comprising 25 percent sodium citrate and 30 percent phenol as a hydrotropic solubilizing agent.
36251
References
- Maheshwari RK. (2009) Solubilization of ibuprofen by mixed solvency approach. Indian Pharm 8:81-84.
- Pareek V, Tambe S, Bhalerao S, Shinde R, Gupta L. (2010) Spectrophotometric estimation of cefprozil by using Different hydrotropic agents. Int J Pharm Pharm Sci 2:45-48.
- Bhawsar N, Maheshwari RK, Ansari A, Saktawat Y (2011) New spectrophotometric estimation of gatifloxacin in the tablets using mixed solvency approach. Int J Pharm Sci 2:270-74.
- Maheshwari RK, Upadhyay N, Jain J, Patani M, Mathuria KC (2011) New spectrophotometric estimation of naproxen tablet formulation employing mixed solvency concept (at 331 nm). Int J Pharm Technol 3:3618-623.
- Patil DN. (2012) Spectroscopic determination of lovastatin by hydrotropic solubilization technique. Int J Pharm Chem Sci 5: 23-27.
- Jayakumar C, Morais AB, Raja C, Gandhi NN (2013) New quantitative estimation of famotidine using hydrotropic solubilizing agents. Int J Pharm Pharm Sci 5: 249-252.
- Soni LK, Solanki SS, Maheshwari RK (2014) Solubilization of poorly water-soluble drug using mixed solvency approach for aqueous injection. J Pharm Res 4:549-68.
- Ruth BV, Kumar AS (2014) Spectrophotometric determination of poorly water-soluble drug Lanzoprazole using Hydrotropic solubilization technique. Indo Am j pharm 6: 45-49
- Maheshwari RK (2015) Solid as a solvent-Novel spectrophotometric analysis of tinidazole tablets using melted phenol as a solvent. Asian J Pharm Res 5:21-24.
- Jain S, Maheshwari RK, Nema RK, Singhvi I (2017) Development and validation of simple UV spectrophotometric method of quantization of Nifedipine in solid dosage formulation using mixed solvency concept. World J of Pharm Res 6:1014-1021.
- Jain Sanjay, Maheshwari RK, Nema RK, Singhvi I (2017) New spectrophotometric estimation of frusemide in the tablets using mixed solvency concept approach. Int J Curr Adv Res 6:8510-8513.
- Salunke PA, Rakhonde K, Rathod A, Rathod G, Patil S (2017) Development and validation of mixed hydrotropic solubilization method for spectrophotometric determination of Ornidazole in bulk drug and tablet. J Pharm Res 11:1114-1119.
- Jain Sy, Maheshwari RK, Nema RK, Singhvi I (2018) Development and validation of simple UV spectrophotometric method of quantization of Ornidazole in solid dosage formulation using mixed solvency concept. World Journal of Pharmacy and Pharmaceutical Sciences 1:824-832.







