Key words
|
| |
| Alloxan, Cassia auriculata, Glibenclamide, Hyperglycaemia, Lipids |
| |
Introduction
|
| |
| Diabetes mellitus, a metabolic disorder characterised by absolute or relative deficiency of insulin, affects carbohydrate, fat and protein metabolism, leading to hyperglycaemia and dyslipidemia. Increase in the prevalence of diabetes during past twenty years is alarming. Apart from some adverse effects like hypoglycaemia, weight gain etc., the major limitation of insulin therapy is development of resistance to conventional insulins. Though the treatment with newer insulins has certain advantages over that of conventional insulin[1,2] the course of disease by and large, appears to be similar with either treatment. Similarly, the oral hypoglycaemic agents used to treat diabetes are also not without adverse effects. Therefore there is a need to develop newer, safe and cost effective antidiabetic agents that can control blood glucose round the clock so as to arrest the related metabolic complications. In this direction more than twelve hundred plants have been screened for antidiabetic activity on the basis of ethnopharmacology[3]. |
| |
| Plants like Eugenia jambolona[4], Allium cepa[5], Azadirachta indica[6], Brassia oleracia [7], Ganoderma lucidum[8] etc. have been extensively studied as alternatives in diabetes. Cassia auriculata seeds have been reported to have hypoglycaemic activity in ayurvedic literature[9].Juice of cassia flowers, seed powder 2-4 gm/day[10] and water extracts of seeds have been reported to possess hypoglycaemic activity in experimental animals[11].Literature survey indicates the different extracts of seeds have not been explored for hypoglycaemic activity. Therefore present study has been planned to investigate the influence of different extracts of cassia seeds on alloxan induced hyperglycaemia. In the present study, hypoglycaemic and hypolipidemic activity of Cassia auriculata seed extracts in alloxan induced hyperglycaemic rats and the interaction of various seed extracts with glibenclamide were also studied. |
| |
MATERIALS AND METHODS
|
| |
|
Collection and authentication of plant:
|
| |
| Cassia auriculata seeds were obtained from in and around Belgaum in November, when it bloom yellow flowers. The authenticity of the seeds as well as plants was confirmed from Agarkar Research Institute (Pune). |
| |
|
Preparation of various extracts of Cassia auriculata seeds:
|
| |
| The seeds were isolated from pods and then were put in an electric mixer and grinded till a uniform granular powder was formed. The alcohol and petroleum ether extracts were selected based on the polarity (alcohol = polar and petroleum ether = non polar) and were prepared by using standard methods. Water extract was taken by the method found in the ayurvedic literature known as Kashaya Kalpana (Decoction) [12] and was prepared freshly every day before administration to rats. About 200 mg of seed powder was packed in soxhlet extractor with 600 ml of 95% v/v ethanol at the temperature 600 C to get the ethanolic extract. The filtered extract was concentrated by rotary evaporator and dried in dessicator over sodium sulphite. After drying, the extract was weighed and percentage extractive value was determined. |
| |
|
Preliminary phytochemical screening:
|
| |
| The preliminary phytochemical analysis of alcoholic, petroleum ether and water extracts was carried out for the Flavonoids (Ferric chloride, Shinoda, Zinc hydrochloric acid reduction, Alkaline reagent and Lead acetate tests), Lipids, Saponins (Foam, Haemolysis tests), Sterols (Salkowski, Liberman-Burchardt & Sulphur tests), Tannins (Ferric chloride, Gelatine tests), Triterpenoids (Salkowski, Liberman-Burchardt tests)(Table I)[13]. |
| |
|
Acute toxicity studies:
|
| |
| The development of a pharmaceutical drug is a stepwise process involving an evaluation of both the animal and human safety information(ICH guidelines).The toxicity studies on all the three extracts were done using OECD (organisation for economic cooperation and development) guidelines for acute oral toxicity. The principle of the test is based on a stepwise procedure with the use of a minimum number of animals per step. Sufficient information is obtained on the acute toxicity of the substance to enable its classification according to Globally Harmonized System (GHS).The substance is administered to the set of three female rats at predetermined doses e.g.(2000 mg/kg body weight for limit test).Absence of the compound related mortality will determine the next step. Since, no mortality with this dose was seen, 1/10th of this dose was used in the subsequent study[14]. |
| |
|
Animals:
|
| |
| The complete course of experiments was carried out using healthy male rats of Wistar strain, weighing between 150-200 g. The animals were acclimatized to normal laboratory conditions with 12:12-h light-dark cycle and were maintained on standard laboratory diet (Amrut feeds) with free access to water. |
| |
| Hyperglycaemia was induced by injecting alloxan monohydrate (Sigma Chemical Co., USA) in single dose of 60 mg/kg (freshly dissolved in 0.3ml of saline) through the tail vein. After 48 h of alloxan treatment, glucose was estimated in the tail vein blood with the help of a glucometer (Pulsatum), and animals with blood glucose ≥200 mg/dl were included in the study. |
| |
|
Drugs used and their doses:
|
| |
| 240 mg of extract was suspended in 2%w/v NaCMC (Sodium carboxy methyl cellulose) and volume was made up to 6ml per group for administering so that volume of administration did not exceed 0.5 ml/100gm rat. Glibenclamide (IP grade powder, courtesty Hoechst Marrion-Roussel, Mumbai) in its pure form was suspended in 2% w/v NaCMC [Stelzergi] and administered in the dose of 0.9 mg/kg. All the three extracts of seeds of Cassia auriculata were administered in the dose of 200 mg/kg. In combination studies, all the three extracts of seeds of Cassia auriculata (100 mg/kg) were administered separately in combination with subhypoglycaemic dose (SHD) of glibenclamide 0.45 mg/kg [8] in designated groups while the control group received saline. |
| |
|
Diabetic Studies:
|
| |
| The hyperglycaemic rats were divided in to saline (control) and glibenclamide-0.9 mg/kg (standard) and other six groups(n=6 in each) to receive different extracts of Cassia auriculata viz., alcohol extract in the dose of 200mg/kg (ACA-2), petroleum ether extract 200mg/kg(PCA-2), water extract 200mg/kg(WCA-2) individually as well as combination of these extracts in the dose of 100mg/kg ie.,ACA-1 or PCA-1 or WCA-1 with SHD of glibenclamide. The different treatments were administered once daily orally at 8:00 am for 45 days. After 24 hrs of last dose, under ether anaesthesia 2-3 ml of cardiac blood was collected to estimate glucose by glucose oxidase/ peroxidase (GOD/POD) method using a standard kit (Beacon Diagnostics, India). Blood lipid profile was assessed by estimating cholesterol and HDL cholesterol with the help of cholesterol oxidase/ peroxidase kit (Beacon Diagnostics, India). The blood level of triglycerides were estimated by using a separate kit (Beacon Diagnostics, India) and VLDL, LDL levels were calculated by using the formula: VLDL = Triglycerides/5 and LDL = Total cholesterol – HDL – VLDL [8]. |
| |
| Histopathology of pancreas sections in untreated animals and those treated with alcohol and petroleum ether extracts were stained with haematoxylin and eosin (H&E) and were observed under light microscope (40X) for the presence of islets of beta cells. |
| |
| At the end of experiments the body weights of each animal was noted and the same was compared with that of pre-treated values. |
| |
| All the procedures were performed in accordance with the CPCSEA (Committee for the Purpose of Control and Supervision on Experiments on Animals) guidelines under Ministry of Animal Welfare Division, Government of India and the study was approved by IAEC (Institutional Animal Ethics Committee). |
| |
|
Statistical Analysis:
|
| |
| Data were expressed as Mean ± S.E.M. and were analysed by using one way ANOVA followed by Dunnet’s test. P≤0.05 was considered to be significant. |
| |
RESULTS
|
| |
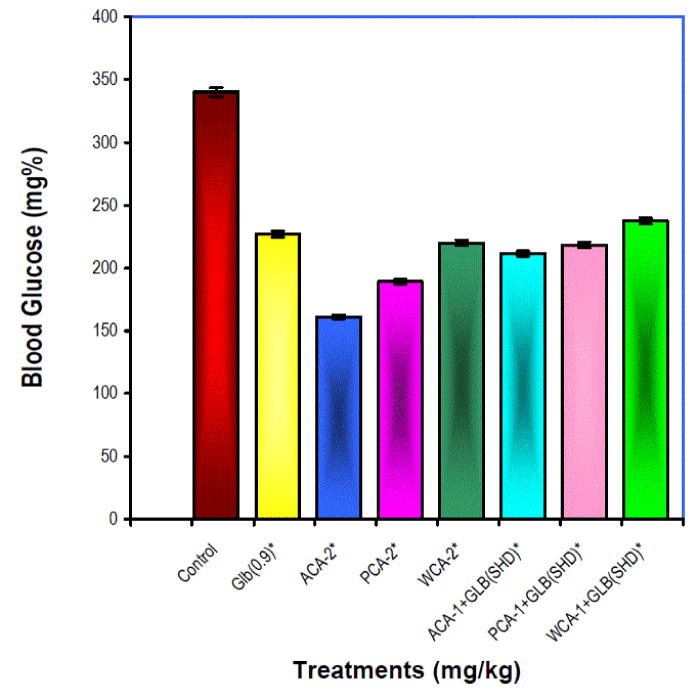
| In chronic studies, 24 hour after the last dose, all the treatment groups produced significant (p<0.01) hypoglycaemia when compared with their pretreatment glucose levels (not shown) (Fig I). Glibenclamide (0.9 mg/kg) raised HDL and lowered triglyceride and VLDL levels significantly (p<0.05) when compared with that of control values. All other treatment groups raised the HDL and lowered total cholesterol, triglycerides, VLDL and LDL levels significantly (p<0.05, p<0.01) when compared with control values (Table II). |
| |
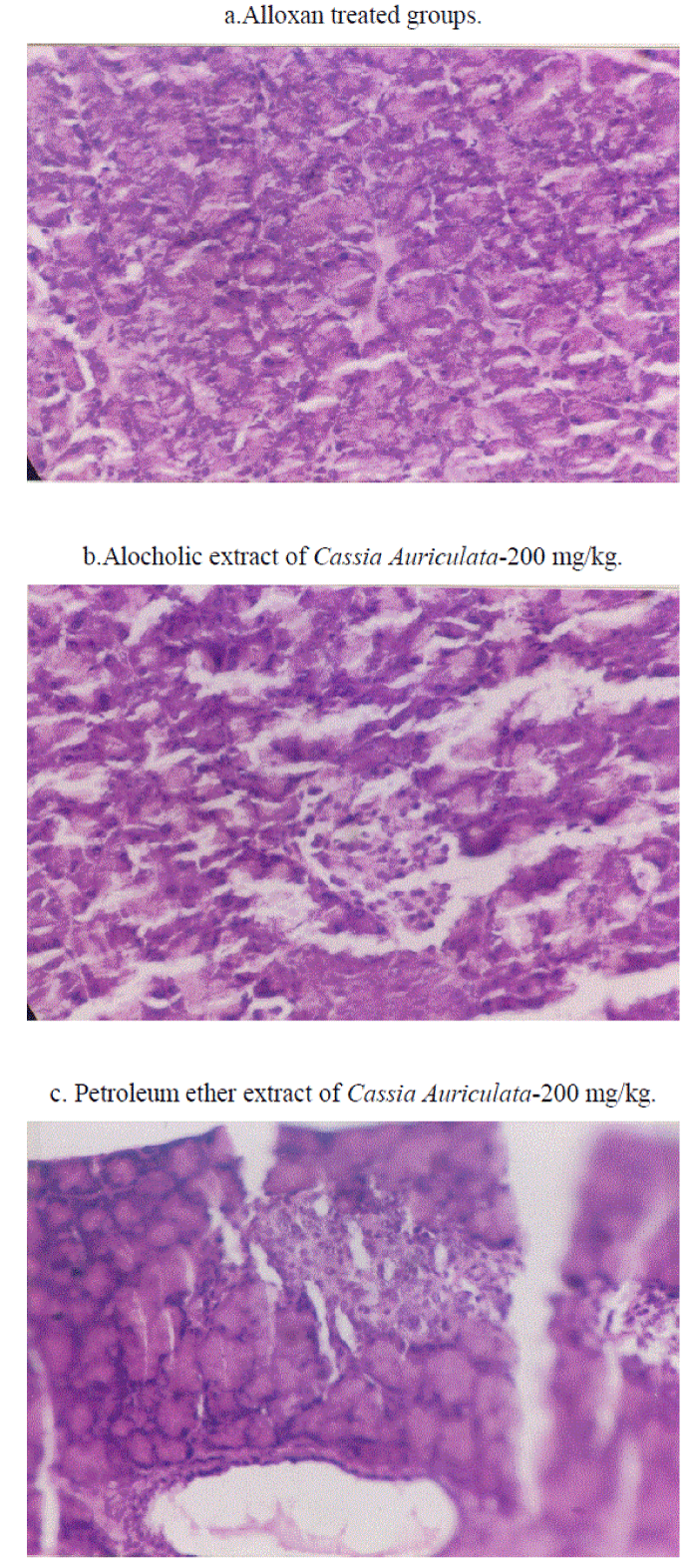
| Microphotographs of histopathological studies showed pancreatic tissue with acini of varying sizes but absence of islets in alloxan treated animals(Fig II-a). In (ACA- 2) treated groups there were pancreatic acini seen along with ill formed islets probably under regeneration (Fig II-b) whereas (PCA-2) treated groups showed pancreatic acini and pancreatic duct as well as well formed islets of langerhans(Fig II-c). |
| |
| Except decreased food intake by ACA-2 and WCA-2 treated groups, there was no significant change in food intake of different treated groups when compared to that of controls. Twenty four hours after the last dose of treatment, significant (p<0.05, p<0.01) weight gain (values not shown) was observed in all the treated groups except combination treatment of PCA-1 with SHD of GLB (+1.05±8.53 g) as compared to their respective pre-treatment weights (0 day).In GLB treated group animals had lost the weight (-7.3?4.22 g) though it was statistically insignificant (p>0.05) where as in saline treated control there was significant (p<0.01) reduction in the bodyweight (-20.76?2.32 g). |
| |
DISCUSSION
|
| |
| All the extracts of Cassia auriculata viz., alcoholic, petroleum ether and water extracts and combination of lower doses of these extracts with SHD of GLB (0.45 mg/kg) have produced significant hypoglycaemia in diabetic animals. Different parts of Cassia auriculata flowers[15,16] and leaves[17,18] have been reported to have hypoglycaemic activity. The reports regarding the hypoglycaemic activity of seed extract are scanty. Also there is paucity of information on the interaction of Cassia auriculata seed extracts with a standard oral hypoglycaemic like glibenclamide. |
| |
| Similarly, all the extracts of Cassia auriculata and combination of lower doses of these extracts with SHD of GLB (0.45 mg/kg) favourably altered the lipid profile in diabetic animals. Cassia auriculata seed extracts have not been studied earlier for their influence on the lipid profile. |
| |
| The mechanism of hypoglycaemic activity of the extracts cannot be proposed by the present study. However a study has indicated that Cassia auriculata flower extract at a higher dose(0.45 g/kg) has decreased gluconeogenic enzymes and increased plasma insulin levels [16].Based on earlier report [19] sitosterols components of petroleum ether extract could be responsible for hypoglycaemic activity [11,20].Beta sitosterol is reported to have insulin releasing effects[21]. |
| |
| In the present study, presence of proanthocyanidins in alcoholic extracts has been shown by HPTLC studies. Such a finding agrees with earlier reports showing the presence of proanthocyanidins in flower and leaf extracts[22].It is very difficult from the present study to attribute the hypoglycaemic activity of ACA-2 to proanthocyanidins present in it. Persistent antihyperglycemic observed on 47th day (3 days after discontinuing the treatment) which was observed in the present study, indirectly indicates protective effects on beta cells against hyperglycaemia induced damage due to antioxidant effect of proanthocyanidins as reported earlier[22,23].Such proposition is further supported by histopathological findings in the present study i.e.,the presence of pancreatic acini and illformed islets denoting pancreatic regeneration(Fig II). However it is difficult to comment about pancreatic acini preserved/or regenerated are due to treatment with extracts. Hypoglycemic activities of low dose extracts when administered with sub effective dose of glibenclamide could be possibly due to pharmacodynamic interaction of additive nature. |
| |
| The restoration of altered lipid profile to normal in diabetic animals treated with petroleum ether of Cassia auriculata extracts could be due to the sitosterols. Sitosterols are hypocholesterolemic and used in the treatment of atherosclerosis[24]. They are poorly absorbed and compete with cholesterol for absorption sites in the intestine. |
| |
| Hypolipidemic activity of various extracts in low dose when coadministered with SHD of GLB could be probably due to pharmaocodynamic interaction. It is difficult to propose the mechanism of hypolipidemic activity of ACA, WCA which don’t contain sitosterols as compared to petroleum ether extract. Because of close relationship between carbohydrate and lipid metabolism, altered glucose metabolism as in diabetes could be responsible for hyperlipidemia observed in diabetes. Correction of glucose metabolism therefore could restore the altered fat metabolism to normal as observed in humans. Increase in the serum HDL appears to be secondary to the effects of euglycaemia. Such phenomenon has been observed in diabetic patients whose blood sugar was well controlled [25,26,27,28]. |
| |
| Significant gain in the bodyweight, decreased food intake (by ACA-2,WCA-2) and decreased mortality in various extract treated diabetic animals as compared to untreated and glibenclamide treated animals indicate potential benefit of Cassia auriculata extracts in diabetic individuals. The extract treated groups viz., ACA-2 and PCA-2 exceed the hypoglycaemic activity of glibenclamide treated group. Further hypoglycaemic and hypolipidemic principles from the Cassia auriculata extracts need to be identified. |
| |
CONCLUSIONS
|
| |
| Findings of the present study clearly indicate the hypoglycaemic and hypolipidemic properties of Cassia auriculata seed extracts in hyperglycaemic animals. The study also indicates the synergistic interaction of Cassia auriculata seed extracts with clinically used oral hypoglycaemic agents like Glibenclamide and also at the same time cautions about the potential hypoglycaemia that may be involved with coadministration of these two. Clinical studies in this regard are really worthwhile. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
|
|








