Key words
|
| |
| Nimesulide, β-cyclodextrins, kneading, stability. |
| |
Introduction
|
| |
| Nimesulide is described chemically as N-(4-Nitro-2- phenoxyphenyl) methanesulfonamide is a non-steroidal anti-inflammatory agent that selectively inhibits cyclooxygenase-2 (COX-2) [ 1] is used for a variety of acute and chromic inflammatory diseases. It differs from other nonsteroidal anti-inflammatory drugs (NSAIDs) in that its chemical structure contains a sulfonanilide moiety as the acidic group rather than a carboxylic group. Nimesulide shows high antiinflammatory, antipyretic, and analgesic activities in addition to low toxicity, a moderate incidence of gastric side effects, and a high therapeutic index.[ 2] However, recent findings reported that nimesulide has a higher risk of hepatic toxicity when compared to other marketed NSAIDs. [ 3] Like many nonsteroidal antiinflammatory drugs, nimesulide is very sparingly soluble in water (~ 0.01 mg/mL). [ 4] The poor aqueous solubility and wettability of nimesulide gives rise to difficulties in pharmaceutical formulations for oral or parenteral delivery, which may lead to variable bioavailability. Cyclodextrins (CDs) are able to form inclusion complexes with poorly water-soluble drugs. They are often depicted as hollow truncated cones with primary and secondary hydroxyl groups orientated outwards. As a result, CDs have an electron rich hydrophobic internal cavity and a hydrophilic exterior [5]. This unique cavity enables CDs to accommodate a wide range of non-polar molecules via the formation of reversible non-covalent inclusion complexes. CDs not only offer protection to the encapsulated molecule from the outer environment but also improve properties such as bioavailability, stability, solubility, dissolution rate, bioavailability and taste masking of the drug [6-8] . |
| |
| Cyclodextrins can also be used to prevent drug-drug and drug additive interactions, convert liquid drugs into microcrystalline powder, decrease volatility, modify gastrointestinal or ocular irritation and mask of objectionable taste or odor of drugs [5]. Present study shows the effect of β -cyclodextrin on physical stability of nimesulide suspension which also includes formulation and evaluation. |
| |
MATERIALS AND METHODS:
|
| |
| Nimesulide (Acto pharmaceuticals ltd, Warangal), β- cyclodextrin, sodium carboxy methyl cellulose (sodium CMC), sorbitol, methyl paraben sodium, and propyl paraben sodium (Loba chem., Mumbai), sucrose (S.D. fine chemicals, Mumbai), tween 80 and acetone (Merck, Mumbai). |
| |
EXPERIMENTALS
|
| |
|
Preparation of binary mixtures of nimesulide / β – cyclodextrin
|
| |
| Various techniques for the preparation of drugcyclodextrin complexes include co-precipitation[9], slurry complexation (kneading method) [10], paste complexation, damp mixing and heating, extrusion, dry mixing, neutralization, freeze drying and slugging methods. |
| |
|
Complexation with β-cyclodextrins by kneading technique [10]
|
| |
| The nimesulide and β-cyclodextrins complexes (1:0.5, 1:1 and 1:1.5) were prepared by kneading technique. In this method required amount of drug and β- cyclodextrin were taken and transferred to a mortar pestle. The mixture was size reduced by continuous stirring with pestle. Water-ethanol mixture (3:1) ratio was added to the above physical mixture and continuously stirred until the slurry mass was formed. Slurry mass was collected and dried in a hot air oven for 2 hrs at 50°C, dried mass was collected and further dried in desiccators over silica gel for 24 hrs to remove all the excess residual solvents. The dried mass was collected and passed through 60 # mesh, and packed it in a closed container. |
| |
|
Preparation of suspensions:
|
| |
| Suspensions containing 10mg/ml of nimesulide were prepared as per the formula given in Table 1 in about 100 ml of purified water, the required amount of suspending agent (sodium CMC) [11] was added and kept overnight for proper hydration. This solution was used as the vehicle in the preparation of the suspension. Accurately weighed quantity of the drug was distributed in the vehicle. Tween 80 was added to above dispersion. The slurry concentrate of the drug was mixed gently for 15 min. Other ingredients like sorbitol, methyl paraben and propyl paraben were added and the volume was made up with the water. The prepared suspensions were homogenized and transferred to the final containers. |
| |
EVALUATION
|
| |
| Sedimentation Volume [12,13]: |
| |
| Sedimentation volume (F) is nothing but a ratio of the final volume of sediment (Vu) to the original volume of sediment (V0) before settling. 50ml of each suspension were transferred to 50 ml. measuring cylinders and the volume of sediment formed was noted after 24 hr, 2 wk, 3wk, 1 month, 2 month and 3 month. The sedimentation volume (F) was calculated using the formula- |
| |
| F = Vu / Vo |
| |
|
Redispersibility:
|
| |
| The bottles containing suspension were held up right between the fingers and rotated clockwise upside down through 180 °in a semicircular path and back in the anti-clock wise direction (one cycle). This process was repeated continuously until the sediment was completely redispersed. |
| |
|
pH measurement:
|
| |
| The pH of the prepared suspensions was measured by using ELICO INDIA pH analyser (Model LI612) by calibrating with standard buffers (pH 4.2 and pH 9.0). |
| |
|
Viscosity Measurement
|
| |
| The viscosity of the prepared suspensions was measured by Brookfield viscometer (Model: DV- I+) using spindle S-61 at 12 rpm. |
| |
|
Particle size measurement:
|
| |
| The particle size of nimesulide particles in the prepared suspensions was measured by optical microscopy using a trinocular microscope at 100x (10×10) magnification. The size of 100 particles were measured and the average particle size of was determined |
| |
|
Drug content estimation:
|
| |
| 5 ml of the suspension was measured accurately and transferred into a 100ml volumetric flask. Sufficient quantity of 0.1N Sodium hydroxide was added to dissolve the drug and the volume was made up with 0.1N Sodium hydroxide. From this solution, 10 ml was taken and transferred to a 100ml volumetric flask the volume was made up to the mark with 0.1N sodium hydroxide. From this 1ml was drawn into 10 ml volumetric flask and the volume was adjusted with 0.1 N Sodium hydroxide. The absorbance of the solution was measured at 395nm on a UV-Vis spectrophotometer (ELICO SL-159) using 0.1N sodium hydroxide as a blank. |
| |
|
DSC Studies:
|
| |
| The interaction between nimesulide and β- cyclodextrin was characterized by differential scanning calorimetry (DSC). DSC patterns of samples were obtained with Shimadzu DSC-50 instrument using vented aluminium pans. |
| |
RESULTS AND DISCUSSION
|
| |
| Suspension F1 was formulated employing drug and suspending agent, whereas suspensions F2, F3 and F4 were formulated by employing drug, suspending agent and β -cyclodextrin. The drug and β -cyclodextrin complexes in F2, F3 and F4 were in the ratio of 1:0.5, 1:1 and 1: 1.5 respectively. These formulations were evaluated for various quality parameters to determine their stability such as sedimentation volume, pH, viscosity, redispersibility, particle size and drug content for 3 months time in regular intervals (see Table 2, 3, and 4). |
| |
| All formulations contain sodium CMC as the suspending agent. Four suspension formulations were prepared containing sodium CMC in four different concentrations (0.25, 0.5, 0.75 and 1%w/v). These were studied for 48 hours and evaluated for sedimentation volume and redispersibility. The values are presented in Table 5. From the results, it is concluded that, suspension containing 0.75 % w/v sodium CMC shown highest sedimentation volume and less number of cycles for redispersibility. Hence, sodium CMC in the concentration of 0.75%w/v was used in all the suspensions for further studies. |
| |
|
Sedimentation volume:
|
| |
| The sedimentation volume in case of F1 was found to be 0.13 at the end of 24 hours whereas it was around 1.0 in case of all the suspensions containing different ratios of β - cyclodextrin (see Table 2) and with aging it was found that, in F2 and F3 it gradually decreased to 0.442 and 0.513 whereas in case of F4 it was decreased to 0.78. Thus, highest sedimentation ratio was seen with the suspension containing highest ratio of β - cyclodxetrin (1:1.5). This indicates that suspensions containing β -cyclodextrin displayed more stability than those without β -cyclodextrin. |
| |
|
Redispersibility:
|
| |
| Good redispersibility was seen in the case of nimesulide suspensions containing different ratios of β - cyclodxetrin within a 3-4 cycles during the storage (F2, F3 and F4). On the contrary, it took 6-7 cycles in case of F1 (see Table 3). The above conclusion in sedimentation volume holds true here also. |
| |
|
pH:
|
| |
| The pH value of F1 was found to be 8.36 but when the drug was complexed with β - cyclodextrin the values were found to be less ranging from 5.12-6.48 (see Table 3). But on ageing, the values remained more or less constant in all the formulations. This indicates that though complexation results in lowering of pH but there is no chemical change that results on aging. |
| |
|
Viscosity:
|
| |
| The viscosity of F1, F2, F3 and F4 was found to be 26.05, 34.1, 44.5 and 52.5 cps respectively at the end of 24 hours. On ageing, the viscosity of F1 showed no significant change (26.05 -23.9) whereas in case of F2, F3 and F4 the values were decreased (see Table 4). Among these three formulations the change in viscosity in case of F4 was less indicating that F4 is a stable formulation. |
| |
|
Particle size measurement:
|
| |
| There was no significant change in particle size in all the four formulations (F1, F2, F3 and F4) at the end of 24 hours (see Table 4). But on ageing, the particle size marginally increased in case of F1 whereas in all other three cases the size was decreased. The reason for this behavior may be attributed to crystal growth in case of F1 whereas in other cases, as β- cyclodextrin solubilize the drug particles, the size of the particles may be reduced with time. |
| |
|
Drug content:
|
| |
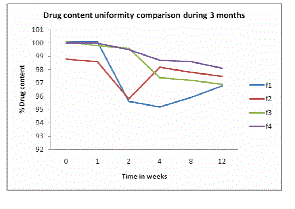
| Assay of the drug shown 95% to 101% nimesulide content indicates that the drug content remains within the standard limits during 3 months (see Figure 1). |
| |
|
DSC studies:
|
| |
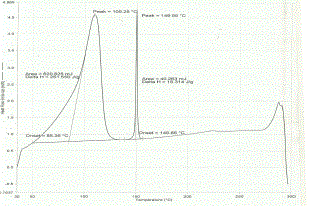
| The thermal behavior of pure drug (nimesulide), β - cyclodextrin and drug- β - cyclodextrin complex was studied using differential scanning calorimetry in order to assess the formation of complex (see Figure 2). The DSC thermogram of nimesulide exhibited an endothermic peak at 150.85oC corresponding to its melting point. β – cyclodxetrin has shown a broad endothermic peak at 114.76 oC, corresponding to its dehydration. The thermogram of the binary mixture contained both the peaks of nimesulide and β - cyclodextrin, wherein the intensity of the drug peak has diminished. This indicates that drug was not completely complex. As nimesulide is a practically insoluble drug, complexation might not have occurred completely. |
| |
CONCLUSION
|
| |
| The present study was carried out to study the effect of β -cyclodxetrin on the physical stability of suspensions. Nimesulide were selected as model drugs and sodium CMC was used as the suspending agent and was used in the concentration of 0.75% w/v in all the formulations as it had shown good results. Inclusion complexes of drug and β - cyclodextrin were prepared in three different concentrations (1.0:0.5, 1.0:1.0 and 1: 1.5) by kneading method.[12] All the suspensions formulated by employing drug- β - cyclodextrin binary systems have shown improvement in physical stability when compared to control (suspension prepared without β - cyclodextrin). The suspensions with β - cyclodextrin exhibited good redispersiblity within a 3-4 cycles during the storage, whereas, more number of cycles are required in case of nimesulide suspensions without β -cyclodextrin. The results of the study indicated inclusion of β- cyclodextrin in the form of complex in suspensions improved the physical stability. Higher the concentration of β - cyclodextrin, better is the stability. In this study, drug- β - cyclodextrin in the ratio of 1:1.5 was found to be optimum. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








