Keywords
Electrical muscle stimulation; ICU; Early rehabilitation; NMES; Frequency
Introduction
A large majority of patients admitted to the intensive care unit (ICU) after the very acute phase of critical illness exhibit major defects in skeletal-muscle strength (weakness) and mass (wasting). ICU acquired weakness (ICUAW) is generally defined as a bilateral deficit of muscle strength in all limbs, which is accompanied by profound loss of muscle mass, as high as 5% per day during the first week of ICU stay [1] and is associated with delayed weaning from mechanical ventilation [2], protracted and costly ICU stay and high mortality rate [3]. Early rehabilitation is emerging as an important means to prevent ICUAW, facilitate and improve long-term recovery and functional independence of patients, and shorten the duration of ventilation and hospitalization [4]. Neuromuscular electrical stimulation (NMES), a means of early rehabilitation, involves the application of an electric current through electrodes placed on the skin over the targeted muscles to induce skeletal muscle contractions.
In ICU- or in-hospital patients, NMES has been shown to preserve protein synthesis and prevent muscle weakness, while it has also the potential to prevent muscle atrophy [5-12]. Acutely applied NMES can induce systemic beneficial effects on microcirculation and endothelial function [13-15]. Evidence suggests that NMES can reduce ICUAW incidence and duration of weaning [16]. NMES has been also shown to be beneficial in other categories of critically ill patients, such as patients with chronic heart failure (CHF) [17-20] and chronic obstructive pulmonary disease (COPD) [21-29].
In the aforementioned studies, protocols with different NMES characteristics have been employed, including frequency (the number of electrical pulses delivered per second), which has been ranged between 10 and 100 Hz. Based on frequency, currents can be categorized as high-frequency (>60 Hz), medium-frequency (30-55 Hz), and low-frequency NMES (<25 Hz). In general, increasing frequency, higher tetanic force output is produced, and higher peak torque is expected [30], which in turn may affect NMES-induced stimulus on muscle mass. However, there are not any data on the effects of differentfrequency NMES protocols on muscle mass in ICU patients.
It was hypothesized that a high-frequency protocol could induce better outcome in terms of muscle wasting than a medium-frequency program. The aim of this pilot study was to explore the effects of two different NMES protocols on muscle mass preservation in critically ill patients during ICU stay.
Methods
Study design and randomization
The present study was a prospective, randomized study conducted in a multidisciplinary ICU. The protocol of the study was approved by the Ethics Committee of the Hospital. Written informed consent was obtained from all patients ’ closest relative.
All patients admitted to the ICU during the study period were considered for inclusion. Inclusion criterion was mechanical ventilation for more than 24 hours, with prediction of hospitalization ICU for more than two days.
Exclusion criteria were: age <18 years, obesity (Body Mass Index (BMI) >35 kg/m2, ICU stay >7 days (patient not on mechanical ventilation) prior to enrollment, mechanical ventilation >48 hours (at the ward) before ICU admission, pregnancy, fracture in the pelvis or legs, a history of neuromuscular disease or muscle weakness (prolonged stay on bed before hospital admission), a pacemaker or defibrillator, moribund >90%, non-applicability of NMES to the lower extremities, history of serious mental/psychological disorder, patient’s closest relative refusal.
At ICU admission patients who met inclusion criteria were randomized after stratification to one of two NMES intervention groups. Stratification was made upon age (≤ or >50 years of age, median value of our ICU patients' age) and severity according to Sequential Organ Failure Assessment (SOFA) score (< or ≥ 8). Randomization was performed by an investigator who was not involved in outcome assessment and patient follow up.
Intervention
NMES implementation was additional to the usual care. Daily sessions (different characteristics, as detailed below) were performed until 10 days after ICU admission. A lightweight, battery-powered stimulator unit Rehab 4 Pro (Cefar Medical AB, Malmö, Sweden) was used which produced a controlled contraction and relaxation of the underlying muscles via selfadhesive electrodes. Patients assigned to intervention groups received daily (7 times/week) NMES sessions by different individual characteristic, as follows:
Medium Frequency (MF) protocol consisted of pulses at 45 Hz frequency, 400 μsec pulse duration, duty cycle of 12 sec on (including 0.8 second rise time and 0.8 second fall time) and 6 seconds off, according to a previous study [16].
High Frequency (HF) protocol consisted of pulses at 75 Hz frequency, 400 μsec pulse duration, duty cycle 5 sec on (including 1.6 sec ramp up and 0.8 sec ramp down) and 21 sec off, based on previous data [5,31].
The duration of the session was 45 minutes for both protocols including 5 minutes for warm up and 5 minutes recovery phase using 10 Hz current of 400 μsec pulse duration. The NMES electrodes (9 X 5 cm) placed on motor point of the quadriceps muscle and the long peroneal muscle after skin cleaning. During the sessions, the angle at the patients ’ knee joint was approximately 30°-40° (0° corresponds to full extension of the knee). The stimulator delivered biphasic, symmetric trapezoid impulses at intensities able to cause visible contractions and be tolerated by the patients. In case of doubt, contraction was confirmed by palpation of the muscle involved. Current intensity, optimally aiming at full muscle contraction, was continuously increased during the sessions, to prevent fatigue. In sedated patients, starting intensity was set at 80% of that resulting in maximal response and increased by 10% every 15 min up to 100%. In non-sedated patients, intensity was initially set to the maximum tolerated level and was increased by 10% (or less in case of discomfort) every 15 min throughout the session. During the sessions, qualitative scores were employed to rate the quality of contractions (0: no contraction, 1: palpable contraction, 2: visible contraction, 3: slight knee extension, 4: full knee extension) and the presence of edema (0: no edema, 1: barely detectable impression when finger is pressed into skin, 2: indentation ≤ 15 sec to rebound, 3: indentation ≤ 30 sec to rebound, 4: indentation >30 sec to rebound) [32].
Assessment of muscle mass
The assessment of muscle mass was done by ultrasonography. Muscle layer thickness of the quadriceps muscle (vastus intermedius and rectus femoris) was measured. Quadriceps selection was based on the fact that it is easily accessible and correlates well with the lean muscle mass [33]. Ultasonogragraphy images were taken the day of randomization (second day of admission), ten days after first assessment and at ICU discharge. Muscle layer thickness of the vastus intermedius and the rectus femoris was assessed bilaterally in the middle of the distance between the anterior superior iliac spine and the midpoint of the patella, with the patient in the supine position and the legs relaxed lying flat in extension. All the ultrasound examinations were performed on GE Vivid 7 Model ultrasound scanner, using a 7.5 MHz transducer with a 5 cm linear array footprint, by a single operator who was blinded to the randomization and not involved in the data analysis. To ensure repeatability, the exact location of the measurement was marked by a permanent marker. Evaluation was also made by a blinded researcher.
Statistical analysis
Normality of distribution was checked with the Shapiro-Wilk test. Differences between groups over time were assessed with factorial analysis of variance (ANOVA) 2 Χ 2 (time Χ group). Within-group differences were assessed with paired samples ttest or Wilcoxon signed-rank test (in case of not normal distribution). Between-group differences were evaluated with ttest for independent samples or Mann-Whitney signed-rank test, as appropriate. Categorical variables were compared with the chi-square test. Statistical significance was set to p<0.05. Continuous variables are reported as mean ± standard deviation (SD). Statistical analyses were performed with IBM SPSS 25 software.
Results
One hundred forty-seven patients satisfied inclusion criteria and were enrolled to the study. One hundred thirty patients were excluded and 17 were finally randomized to the NMES intervention groups (Figure 1). Eight patients of the MF group (images of 2 more patients in relation to left leg were not able to be evaluated, due to technical reasons) and four patients in the HF group were finally evaluated. Baseline characteristics of the patients finally evaluated are presented in Table 1.
| |
Age |
Gender |
SOFA |
APACHE II |
SAPS III |
Post-surgery patient |
Diagnostic category |
Comorbidities^ |
| MF group |
| 1 |
65 |
F |
11 |
22 |
65 |
Yes |
Sepsis |
Respiratory, Cardiovascular |
| 2 |
51 |
M |
7 |
15 |
49 |
Yes |
Trauma |
Respiratory, Diabetes |
| 3 |
74 |
M |
13 |
25 |
83 |
No |
Respiratory |
None |
| 4 |
45 |
F |
6 |
10 |
59 |
Yes |
Neurological |
Other |
| 5 |
55 |
F |
7 |
21 |
76 |
No |
Neurological |
Gastrointestinal, hepatic |
| 6 |
20 |
M |
6 |
8 |
65 |
Yes |
Trauma |
None |
| 7 |
57 |
M |
9 |
18 |
55 |
No |
Neurological |
Other |
| 8 |
77 |
F |
4 |
15 |
68 |
No |
Respiratory |
Cardiovascular, Other |
| Mean ± SD |
56 ± 16 |
|
8 ± 3 |
17 ± 6 |
65 ± 11 |
|
|
|
| HF group |
| 1 |
47 |
M |
4 |
13 |
56 |
No |
Sepsis |
Other |
| 2 |
87 |
F |
6 |
4 |
64 |
No |
Respiratory |
Cardiovascular, Diabetes |
| 3 |
48 |
M |
6 |
8 |
34 |
Yes |
Trauma |
None |
| 4 |
76 |
M |
10 |
15 |
58 |
Yes |
Neurological |
Respiratory |
| Mean ± SD |
65 ± 20 |
|
7 ± 3 |
10 ± 5 |
53 ± 13 |
|
|
|
MF: Medium Frequency; HF: High Frequency; F: Female; M: Male; SOFA: Sequential Organ Failure Assessment; APACHE: Acute Physiology and Chronic Health Evaluation; SAPS: Simplified Acute Physiology Score.
^9 categories of comorbidities were considered: Respiratory disease, Cardiovascular disease, Diabetes mellitus, Gastrointestinal disease, Haematological disease, Haepatic disease, Renal disease, Other, None
Table 1 Demographic and clinical characteristics of patients at ICU admission.
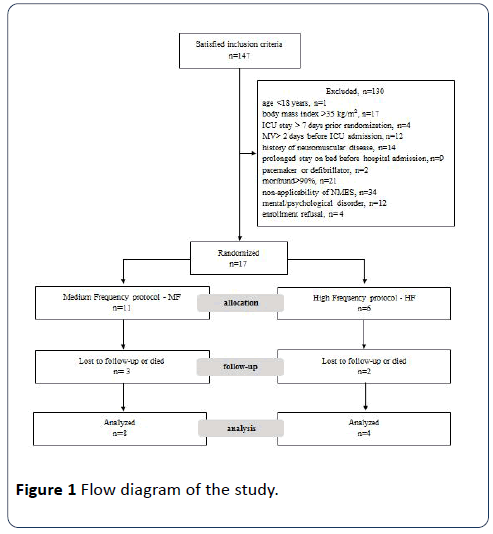
Figure 1: Flow diagram of the study.
No difference (p>0.05) between MF and HF groups (respectively) was observed for age (56 ± 16 vs. 65 ± 20 years), gender (4/4 vs. 3/1 male/female), SOFA score (8 ± 3 vs. 7 ± 3), APACHE II score (17 ± 6 vs. 10 ± 5) and SAPS III score (65 ± 11 vs. 53 ± 13) at ICU admission.
In relation to right quadriceps muscle, muscle layer thickness decreased in the MF (from 2.7 ± 0.9 to 2.4 ± 0.7 mm, 9.5 ± 7.2%, p=0.04) and the HF (from 2.7 ± 0.6 to 2.5 ± 0.5 mm, 6.7 ± 3.7%, p=0.05) group. In concern to left quadriceps muscle, thickness was also decreased in the MF (from 2.7 ± 0.8 to 2.2 ± 0.6 mm, 20.2 ± 6.2%, p=0.03) and the HF (from 2.7 ± 0.6 to 2.4 ± 0.7 mm, 15.7 ± 10.5%, p=0.06). For right and left legs (respectively), no significant between-group differences were found for either absolute (p=0.58 and 0.41) or percentage (p=0.50 and 0.41) decrease (Figures 2 and 3).
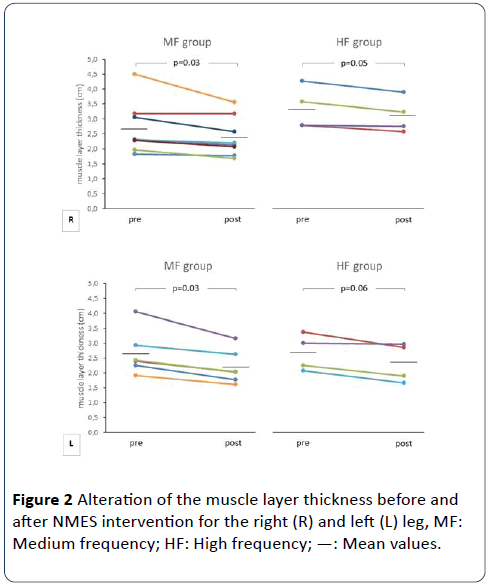
Figure 2: Alteration of the muscle layer thickness before and after NMES intervention for the right (R) and left (L) leg, MF: Medium frequency; HF: High frequency; —: Mean values.
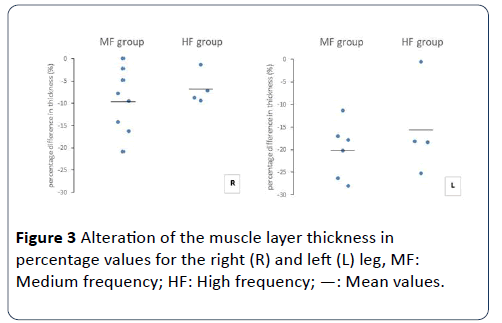
Figure 3: Alteration of the muscle layer thickness in percentage values for the right (R) and left (L) leg, MF: Medium frequency; HF: High frequency; —: Mean values.
No significant differences between the MF and HF groups (respectively) were also observed for number (8.0 ± 1.1 vs. 7.8 ± 1.3 sessions, p=0.73) and percentage (93 ± 8% vs. 97 ± 6%, p=0.46) of NMES sessions completed. That was also the case for strength of contraction (2.5 ± 0.9 vs. 3.5 ± 0.6, p=0.11) and edema (0.2 ± 0.3 vs. 0.0 ± 0.0, p=0.57) (Table 2).
| |
Current intensity of sessions (mΑ) |
Strength of contraction |
Edema |
Sessions |
| Start |
End |
Number |
Percentage |
| MF group |
| 1 |
72 |
85 |
1 |
1 |
9 |
100 |
| 2 |
26 |
32 |
3 |
0 |
9 |
100 |
| 3 |
19 |
24 |
2 |
0 |
8 |
89 |
| 4 |
14 |
18 |
3 |
0 |
7 |
78 |
| 5 |
11 |
15 |
2 |
0.5 |
8 |
89 |
| 6 |
13 |
16 |
3 |
0 |
9 |
100 |
| 7 |
13 |
16 |
4 |
0 |
8 |
89 |
| 8 |
20 |
25 |
2 |
0 |
6 |
100 |
| Mean ± SD |
24 ± 20 |
29 ± 23 |
2.5 ± 0.9 |
0.2 ± 0.3 |
8.0 ± 1.1 |
93 ± 8 |
| HF group |
| 1 |
22 |
26 |
3 |
0 |
9 |
100 |
| 2 |
14 |
18 |
4 |
0 |
6 |
100 |
| 3 |
16 |
20 |
4 |
0 |
8 |
100 |
| 4 |
21 |
26 |
3 |
0 |
8 |
89 |
| Mean ± SD |
18 ± 4 |
22 ± 4 |
3.5 ± 0.6 |
0 ± 0 |
7.8 ± 1.3 |
97 ± 6 |
Table 2 Current intensity of NMES sessions, strength of contraction, edema and sessions performed from ICU admission up to 10 days after.
Discussion
The main finding of our study was that different protocols of NMES implementation showed similar effect on muscle mass of critically ill patients. To our knowledge, this is the first study to compare effects of medium vs. high-frequency NMES on muscle mass in ICU and critically ill patients.
There have been many studies in CHF, COPD and ICU patients, suggesting beneficial effects of NMES on aerobic exercise capacity, cachexia and muscle mass preservation, and quality of life [17-29]. Different NMES protocols have been employed, with frequencies ranging from 10 Hz to 100 Hz; however, there are limited data comparing effects of different frequencies. Sillen et al. [34] showed similar effects of low (15 Hz) vs. high-frequency (75 Hz) on oxygen uptake, ventilation, and symptom perception during a single NMES session on quadriceps muscles in patients with COPD. In an intervention study with severely dyspneic COPD individuals that compared the efficacy of high (75 Hz) and low-frequency (15 Hz) NMES on quadriceps muscle weakness, Sillen et al. observed higher increase in muscle strength and endurance after the high-frequency protocol [24]. Chaplin et al compared changes in muscle strength after NMES of different frequency (50 and 35 Hz) in patients admitted to hospital with an acute exacerbation of COPD, and they did not find any significant difference between groups [35]. These studies, however, are not quite comparable as different levels of current frequency and endpoints have been employed.
The NMES protocols employed in this study were also previously used by our group to investigate the NMES acute effects in the ICU setting. A single session of the lower limbs, with either medium- or high-frequency current, acutely mobilized endothelial progenitor cells, an index of the endothelium restoration potential, and affected local and systemic muscle microcirculation [14,15]. Both NMES currents were similarly effective. Interestingly, strength of muscle contraction was better correlated to changes in microcirculatory variables than current characteristics.
Additionally, in a study with healthy participants, a single session of high-frequency (60 Hz) NMES protocol induced a higher increase in molecular indices of muscle hypertrophy compared to a low-frequency (20 Hz) protocol [36]. Higher frequencies potentially generate higher peak torque than lower frequencies, due to increased twitch summation during muscle contraction [30]. Consequently, they may induce better local muscle adaptations in ICU patients, such as muscle mass preservation and strength, in turn. Benefits related to prevention of muscle wasting have been observed with medium- and high-frequency currents [6-9]. We were not able to observe any differences between MF and HF groups in our study. That was the case not only for muscle mass, but also for strength of contraction; however, these results are underpowered to reach definite conclusion.
Although similar effects on muscle mass were induced, both protocols did not completely alleviate muscle wasting. This finding is in line with some previous studies, but not with others. In the study of Gerovasili et al. [6], NMES was associated with a lower degree of muscle mass loss, as evaluated with ultrasonography and measurements of the cross-sectional area of the vastus intermedius and rectus femoris. In the study of Dirks et al. [7], NMES resulted in no changes on type 1 and 2 muscle fiber cross-sectional area as well as increased phosphorylation of key proteins involved in the regulation of muscle protein synthesis, as evaluated with muscle biopsies. In any case, alleviation of muscle wasting is related to prevention of ICUAW. NMES probably acts as an anabolic stimulus to the muscle, reversing the catabolic effects of critical illness and immobilization [10,11]. Muscle mass is also related, up to some extent, to muscle strength, which has been also shown to improve with NMES intervention [8,9].
The rate of muscle mass loss observed in this study (from 6.7-9.5% to 15.7-20.2%) may be considered comparable to rates previously observed (8% to 12.5%) in another study of our group [6]. Lower rates, within 4%, have been also reported [8]. Beyond sample size, other confounding factors potentially include the evaluation method, the study design and the current characteristics.
A couple of limitations should be mentioned; these were the small sample size and the lack of a no-NMES intervention control group. However, this was a pilot study to provide some preliminary data on the effects of different NMES protocols.
Conclusion
In conclusion, a medium and a high-frequency NMES protocols applied in ICU patients resulted in similar effects on muscle layer thickness of the quadriceps muscle. Further studies are necessary for the optimal frequency to be established.
Acknowledgment
The authors acknowledge the contribution of Dr. Plantza Paraskevi and Dr. Charitidi Maria for their support and assistance.
Funding
This study was partially supported by the Special Account for Research Grants, National and Kapodistrian University of Athens.
Competing and Conflicting Interests
Authors declare that they have no conflicts of interest.
24514
References
- Reid CL, Campell IT, Little RA (2004) Muscle wasting and energy balance in critical illness. Clin Nutr 23: 273-280.
- De Jonghe B, Bastuji-Garin S, Durand MC, Malissin I, Rodrigues P, et al. (2007) Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 35: 2007-2015.
- Nanas S, Kritikos K, Angelopoulos E, Siafaka A, Tsikriki S, et al. (2008) Predisposing factors for critically illness polyneuromyopathy in a multidisciplinary intensive care unit. Acta Neurol Scand 118: 175-181.
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, et al. (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized controlled trial. Lancet 373: 1874-1882.
- Rodriguez PO, Setten M, Maskin LP, Bonelli I, Vidomlansky SR, et al. (2012) Muscle weakness in septic patients requiring mechanical ventilation: protective effect of transcutaneous neuromuscular electrical stimulation. J Crit Care 27: 319.
- Gerovasili V, Stefanidis K, Vitzilaios K, Karatzanos E, Politis P, et al. (2009) Electrical muscle stimulation preserves the muscle mass of critically ill patients: a randomized study. Crit Care 3: R161.
- Dirks ML, Hansen D, Van Assche A, Dendale P, Van Loon LJ (2015) Neuromuscular electrical stimulation prevents muscle wasting in critically ill comatose patients. Clin Sci (Lond) 128: 357-365.
- Fischer A, Spiegl M, Altmann K, Winkler A, Salamon A, et al. (2016) Muscle mass, strength and functional outcomes in critically ill patients after cardiothoracic surgery: does neuromuscular electrical stimulation help? The Catastim 2 randomized controlled trial. Crit Care 20: 30.
- Falavigna LF, Silva MG, Freitas AL, Silva PF, Paiva Júnior MD, et al. (2014) Effects of electrical muscle stimulation early in the quadriceps and tibialis anterior muscle of critically ill patients. Physiother Theory Pract 30: 223-238.
- Strasser EM, Stattner S, Karner J, Klimpfinger M, Freynhofer M, et al. (2009) Neuromuscular electrical stimulation reduces skeletal muscle protein degradation and stimulates insulin-like growth factors in an age- and current-dependent manner: a randomized, controlled clinical trial in major abdominal surgical patients. Ann Surg 249: 738-743.
- Weber-Carstens S, Schneider J, Wollersheim T, Assmann A, Bierbrauer J, et al. (2013) Critical illness myopathy and GLUT4: significance of insulin and muscle contraction. Am J Respir Crit Care Med 187: 387-396.
- Hirose T, Shiozaki T, Shimizu K, Mouri T, Noguchi K, et al. (2013) The effect of electrical muscle stimulation on the prevention of disuse muscle atrophy in patients with consciousness disturbance in the intensive care unit. J Crit Care 28: 536.
- Gerovasili V, Tripodaki E, Karatzanos E, Pitsolis T, Markaki V, et al. (2009) Short-term systemic effect of electrical muscle stimulation in critically iII patients. Chest 136: 1249-1256.
- Stefanou C, Karatzanos E, Mitsiou G, Psarra K, Aggelopoulos E, et al. (2016) Neuromuscular electrical stimulation acutely mobilizes endothelial progenitor cells in critically ill patients with sepsis. Ann Intensive Care 6: 21.
- Angelopoulos E, Karatzanos E, Dimopoulos S, Mitsiou G, Stefanou C, et al. (2013) Acute microcirculatory effects of medium frequency versus high frequency neuromuscular electrical stimulation in critically ill patients - a pilot study. Ann Intensive Care 3: 39.
- Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, et al. (2010) Electrical muscle stimulation prevents critical illness polyneuromyopathy: a randomized parallel intervention trial. Crit Care 14: R74.
- Nuhr MJ, Pette D, Berger R, Quittan M, Crevenna R, et al. (2004) Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J 25: 136-143.
- Maillefert JF, Eicher JC, Walker P, Dulieu V, Rouhier-Marcer I, et al. (1998) Effects of low-frequency electrical stimulation of quadriceps and calf muscles in patients with chronic heart failure. J Cardiopulm Rehabil 18: 277-282.
- Quittan M, Wiesinger GF, Sturm B, Puig S, Mayr W, et al. (2001) Improvement of thigh muscles by neuromuscular electrical stimulation in patients with refractory heart failure: A single-blind, randomized, controlled trial. Am J Phys Med Rehabil 80: 206-214.
- Quittan M, Sochor A, Wiesinger GF, Kollmitzer J, Sturm B, et al. (1999) Strength improvement of knee extensor muscles in patients with chronic heart failure by neuromuscular electrical stimulation. Artif Organs 23: 432-435.
- Vivodtzev I, Pepin JL, Vottero G, Mayer V, Porsin B, et al. (2006) Improvement in quadriceps strength and dyspnea in daily tasks after 1 month of electrical stimulation in severely deconditioned and malnourished COPD. Chest 129: 1540-1548.
- Bourjeily-Habr G, Rochester CL, Palermo F, Snyder P, Mohsenin V (2002) Randomised controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax 57: 1045-1049.
- Neder JA, Sword D, Ward SA, Mackay E, Cochrane LM, et al. (2002) Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD). Thorax 57: 333-337.
- Sillen MJ, Franssen FM, Delbressive JM, Vaes AW, Wouters EF, et al. (2014) Efficacy of lower –limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: results from the DICES trial. Thorax 69: 525-531.
- Abdellaoui A, Prefaut C, Gouzi F, Couillard A, Coisy-Quivy M, et al. (2011) Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J 38: 781-788.
- Zanotti E, Felicetti G, Maini M, Fracchia C (2003) Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest 124: 292-296.
- Gerovasili V (2012) Neuromuscular electrical stimulation appears to be useful in people with severe chronic obstructive pulmonary disease. J Physiother 58: 270.
- Vieira P, Chiappa A, Cipriano G Jr, Umpierre D, Arena R, et al. (2014) Neuromuscular electrical stimulation improves clinical and physiological function in COPD patients. Eur Resp J 44: 58.
- Vivodtzev I, Debigare R, Gagnon P, Mainguy V, Saey D, et al. (2012) Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest 141: 716-725.
- Gorgey A, Mahoney E, Kendall G, Dudley G (2006) Effects of neuromuscular electrical stimulation parameters on specific tension. Eur J Appl Physiol 97: 737-744.
- Gondin J, Brocca L, Bellinzona E, D'Antona G, Maffiuletti NA, et al. (2011) Neuromuscular electrical stimulation training induces atypical adaptations of the human skeletal muscle phenotype: a functional and proteomic analysis. J Appl Physiol 110: 433-450.
- Segers J, Hermans G, Bruyninckx F, Meyfroidt G, Langer D, et al (2012) Feasibility and safety of neuromuscular electrical stimulation on the intensive care unit: preliminary results. Eur Respir J 40: 71s.
- Campbell IT, Watt T, Withers D, England R, Sukumar S, et al. (1995) Muscle thickness, measured with ultrasound, may be an indicator of lean tissue wasting in multiple organ failure in the presence of edema. Am J Clin Nutr 62: 533-539.
- Sillen M, Wouters E, Franssen F, Meijer K, Stakenborg K, et al. (2011). Oxygen uptake, ventilation, and symptoms during low-frequency versus high-frequency NMES in COPD: a pilot study. Lung 189: 2-26.
- Chaplin E, Houchen L, Greening NJ, Harvey-Dunstan T, Morgan MD, et al. (2013) Neuromuscular stimulation of quadriceps in patients hospitalised during an exacerbation of COPD: a comparison of low (35 Hz) and high (50 Hz) frequencies. Physiother Res Int 18: 148-156.
- Mettler J, Magee D, Doucet B (2018) High-Frequency Neuromuscular Electrical Stimulation Increases Anabolic Signaling. Med Sci Sports Exerc 50: 1540-1548.








