Keywords
Nonsteroidal antiinflammatory drugs (NSAIDs); Nanoparticles; RAW264.7 cells; Interleukin-6; Tumor necrosis factor-a; Nitric oxide; Prostagrandin; Cell culture
Introduction
Inflammatory reaction is a body defense reaction that occurs when foreign substances enter the body, or the body is exposed to noxious stimuli such as infection, injury, burns and allergens [1,2]. Although pain due to inflammatory reaction is one of the indispensable reactions that act as danger signals for the body, excessive inflammation has a bad influence on the human body, as it could cause damage and hypofunction of biological tissues and necessitate treatment with anti-inflammatory or other drugs [3]. Inflammatory reaction due to noxious stimuli resolves by repair involving microcirculatory changes, inflammatory cell infiltration and formation of granulation tissue. However, if the repair processes do not progress reasonably or local damage persists, inflammation can become chronic or prolonged. Chemical mediators produced by damaged tissues and inflammatory cells, such as nitric oxide (NO), histamine, prostaglandins (PGs), leukotrienes, platelet-activating factor, cytokines and cell growth factors, cause pain and dysfunction [1,2]. NO is a vascular smooth muscle relaxant factor produced by the vascular endothelial cells, and macrophages have the function of synthesizing NO: cytokines produced at sites of infection and inflammation, such as interleukin (IL)-1β, tumor necrosis factor-α (TNF-α) and interferon-γ (INF-γ), induce the expression of inducible nitric oxide synthase (iNOS), leading to the production of NO [4-6]. NO produced in excess reacts with superoxide to form peroxynitrite, which damages deoxyribonucleic acid (DNA), thereby damaging cells and tissues and exacerbating inflammatory symptoms. Nonsteroidal anti-inflammatory drugs (NSAIDs) have been widely used for the treatment of inflammatory diseases to suppress the inflammatory reactions described above. NSAIDs have excellent antiinflammatory and analgesic effects and are widely used in the treatment of inflammatory diseases, such as chronic rheumatoid arthritis, and chronic musculoskeletal pain, such as low back pain and arthralgia, and for postoperative analgesia. NSAIDs inhibit the cyclooxygenases (COX-1 and COX-2), which play an important role in the conversion of arachidonic acid to eicosanoids [7,8]. Caution is required when NSAIDs are used for long periods of time, because the inhibition of COX-1 in the gastric mucosa causes gastrointestinal toxicity [9,10]. Recent studies have reported that NSAIDs suppress carcinogenesis in the stomach, colon, and other organs through inhibition of COX-2 [11], leading to the expansion of their use.
Nanotechnology is a technology that produces materials with new functions or excellent characteristics by controlling the structure and arrangement of materials at the nano level, and this technology is being actively developed [12]. At present, various nanomaterials are produced using this technology, and products in various fields, such as pharmaceuticals, foods, cosmetics, and chemicals, are already in the market. Nanomaterials are materials with a length of 1 to 100 nm or aggregates/clusters with nanoscale internal structures [13]. In the world of pharmaceuticals, advances in nanotechnology have allowed the development of drug delivery systems (DDS) using nanoparticles or microparticles as carriers. Drug systems using nanoparticles are expected as a strategy to increase drug bioavailability. Nanoparticles improve the bioavailability of orally administered NSAIDs and thereby reduce the required doses of these drugs, which may increase the use of nanoparticles in the treatment of patients with inflammatory diseases. Therefore, in this study, we investigated the influence of the particle size of NSAIDs on the viability of macrophages in the mouse. In addition, we also investigated the influence of the particle size of NSAIDs on the production of NO and inflammatory cytokines as a part of the inflammatory responses of these cells to stimulation with lipopolysaccharide (LPS).
Materials and Methods
Materials
Indomethacin (IMC), ketoprofen (KET), piroxicam (PXC) as bulk powder were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Other reagents used were guaranteed commercial-grade products.
Preparation of fine particles of NSAIDs
NSAIDs were incorporated into fine particles by processing the drug, zirconia beads (2.5 g), and an aqueous polymer (hydroxypropyl cellulose SSL [HPC]) solution in a planetary centrifugal mixer (NP- 100; THINKY Corporation; fitted with a -20°C freezer) at a rotation rate of 1700 rpm, mixing time of 10 min, and medium (zirconia balls) quantity of 2.5 g. The particle size of the drug in the suspension was measured using a laser diffraction particle size distribution meter. The bulk powder (bulk) used in the present study were IMC (IMCbulk), KET (KETbulk), and PXC (PXCbulk), and the fine-particle formulations of these drugs are expressed as IMCnano, KETnano, and PXCnano, respectively.
Cell line and cell culture
RAW264.7 cells were used as the mouse macrophages. RAW264.7 cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ mL of penicillin and 100 μg/mL of streptomycin. The cells were cultured in a CO2 incubator (37°C, 5% CO2).
Evaluation of the toxicity of the NSAID nanoparticles on the mouse macrophages (RAW264.7 cells)
RAW264.7 cells were inoculated into 96-well plates at 1 × 105 cells/ well and precultured for 24 hours. NSAIDs were added to the cells at final concentrations of 100, 50, 20 and 10 μg/mL, and the cells were cultured for 24 hours. A mixture of the cell suspension at 1 × 105 cells/ well and culture medium was used as the control (CTL). After culture, the cells were stained with the MTT Cell Count Kit, and the optical density (OD) was measured using a microplate reader at 570 (630) nm. The OD of each sample relative to that of the CTL was calculated as the cell viability.
Evaluation of the anti-inflammatory effects of the NSAID nanoparticles on LPS-induced acute inflammation in the mouse macrophages (RAW264.7 cells)
RAW264 cells were inoculated into 96-well plates at 2 × 105 cells/ well and precultured for 24 hours. LPS (100 ng/mL, lipopolysaccharide from Escherichia coli O127:B8, Sigma USA) and NSAIDs were added to the cells. The cells were cultured for 20 hours and the measurements were conducted in the culture supernatants. The NSAIDs were used at the final concentrations of 20 and 10 μg/mL. Culture medium was added instead of an NSAID in the control. The amounts of NO, inflammatory cytokines (IL-6 and TNF--α) and prostaglandin E2 were measured to evaluate the anti-inflammatory effects. The amount of NO production was assayed using the Griess method. TNF-α and IL-6 were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc., USA). Prostaglandin E2 was measured using a Prostaglandin E2 Assay Kit. Dexamethasone (DEX) was used as the positive control.
Statistical analysis
The obtained values are shown as the means ± standard error (SE). A one-way analysis of variance (ANOVA) with post hoc test was performed to determine the differences between the groups. The level of significance was 0.05.
Results
Formation of fine particles of NSAIDs
Fine particle formulations of IMC, KET and PXC were successfully created. However, diclofenac sodium could not be made into fine particles because it was soluble in the suspending agent. The particle sizes of the fine-particle NSAIDs are data not shown, the particle sizes were 72, 68 and 75 μm for IMCai, KETai and PXCai, respectively. Particle size measured 14 d post crushing, was unchanged from that recorded immediately after crushing.
Evaluation of the toxicity of the NSAID nanoparticles on the mouse macrophages
Table 1 shows the influence of the particle size of the NSAIDs on the mouse macrophages. The viability decreased in a dose-dependent manner for all NSAIDs. The NSAIDnano decreased the viability of the RAW cells to a significantly greater degree than unprocessed NSAIDbulk at concentrations of 50 and 100 μg/mL. However, at the concentrations of 10 and 20 μg/mL, there were no significant differences in the effect on the cell viability between the NSAIDnanoand unprocessed NSAIDbulk. Comparison among the NSAIDs revealed that PXC at a concentration of 100 g/mL was the most effective at reducing the viability of the RAW cells. The NSAIDs were used at the concentration of 20 μg/mL, at which there were no differences in their effects on the macrophage viability, to evaluate their anti-inflammatory effects.
| |
|
Concentration (mg/ml) |
Cell Viability (%) |
| Bulk Powder |
Indomethacin (IMC) |
10 |
110.0 ± 1.9 |
| 20 |
102.2 ± 2.3 |
| 50 |
97.9 ± 2.7 |
| 100 |
87.7 ± 2.0#,* |
| Ketprofen (KET) |
10 |
105.1 ± 0.3 |
| 20 |
99.7 ± 2.3 |
| 50 |
94.7 ± 2.1* |
| 100 |
87.2 ± 0.2#,* |
| Piroxicam (PXC) |
10 |
102.1 ± 0.5 |
| 20 |
98.5 ± 2.1 |
| 50 |
94.5 ± 3.2#,* |
| 100 |
86.1 ± 0.7#,* |
| Nano Particle |
>Indomethacin (IMC) |
10 |
102.9 ± 1.5 |
| 20 |
98.3 ± 1.3* |
| 50 |
89.7 ± 1.1#,* |
| 100 |
79.7 ± 0.6#,* |
| Ketprofen (KET) |
10 |
102.4 ± 2.0 |
| 20 |
95.7 ± 3.1 |
| 50 |
82.9 ± 1.0#,* |
| 100 |
77.8 ± 1.4#,* |
| Piroxicam (PXC) |
10 |
98.3 ± 3.6 |
| 20 |
94.2 ± 3.0* |
| 50 |
85.6 ± 1.3#,* |
| 100 |
69.2 ± 1.6#,* |
Each value is expressed as mean ± SE (n=3); #Statistically significant differences were seen when compared to the control (non-treated group) (P<0.05); *There were statistically significant differences when compared with the 20 µg/mL group (P<0.05)
Table 1: Cell viability of RAW cells after incubation with different NSAIDs for 24 h.
Evaluation of the anti-inflammatory effects of the NSAID nanoparticles on the LPS-induced acute inflammatory responses in the mouse macrophages
The influence of NSAIDs on the production of NO in the mouse macrophages (RAW264.7 cells) induced by LPS was investigated. As shown in the Figure 1, the RAW 264.7 cells produced NO upon exposure to LPS. Both the unprocessed NSAIDbulkand NSAIDnano significantly suppressed the production of NO by the LPS-stimulated macrophages. NSAIDnano suppressed the production of NO to a significantly greater degree than the unprocessed NSAIDbulk. Among the NSAIDs, IMCnano most strongly suppressed the production of NO. Next, the influence of the NSAIDs on the production of inflammatory cytokines (IL-6 and TNF-α) induced by LPS was investigated (Figure 2). The influence of NSAIDs on IL-6 production is shown in Figure 2A. Both unprocessed NSAIDbulk and NSAIDnano significantly suppressed the production of IL-6, with the NSAIDnano suppressed the production of IL-6 to a significantly greater degree than the unprocessed NSAIDbulk. Comparison among the NSAIDnano revealed that the IL-6 production was significantly lower in the cells treated with IMCnano than in those treated with the other NSAIDnano. Likewise, both unprocessed NSAIDbulk and NSAIDnanosignificantly suppressed the production of TNF-α, with the NSAIDnano suppressed the production of TNF-α to a significantly greater degree than the unprocessed NSAIDbulk (Figure 2B). Comparison among the NSAIDnano showed that TNF-α production also was significantly lower in the cells treated with IMCnano than in those treated with the other NSAIDnano. The influence of NSAIDs on the production of PGE2 is shown in Figure 3. Both unprocessed NSAIDbulk and NSAIDnano significantly suppressed the production of PGE2, with the NSAIDnano suppressed the production of PGE2 to a significantlys greater degree than the unprocessed NSAIDbulk. Comparison among the NSAIDnano revealed that PGE2 production was significantly lower in the cells treated with IMCnano than in those treated with the other NSAID nanoparticles.

Figure 1: Effect of particle size on LPS-induced NO production of each NSAID in RAW264.7 cell. Each value is expressed as mean ± SE (n=3). Each NSAIDs group and DEX group showed a significantly lower value than the CTL group (not show mark). #Statistically significant differences were seen when compared to the each NSAIDbulk (P<0.05). *There were statistically significant differences when compared with the IMCnano group (P<0.05).
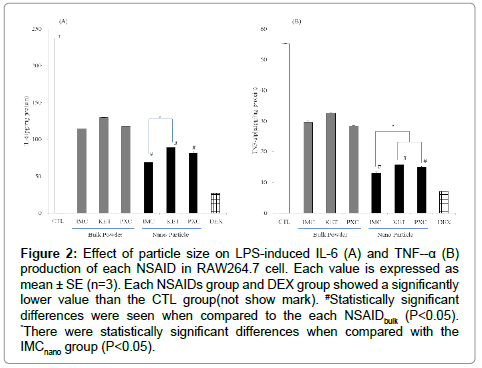
Figure 2: Effect of particle size on LPS-induced IL-6 (A) and TNF--α (B) production of each NSAID in RAW264.7 cell. Each value is expressed as mean ± SE (n=3). Each NSAIDs group and DEX group showed a significantly lower value than the CTL group(not show mark). #Statistically significant differences were seen when compared to the each NSAIDbulk (P<0.05). *There were statistically significant differences when compared with the IMCnano group (P<0.05).

Figure 3: Effect of particle size on LPS-induced PGE2 production of each NSAID in RAW264.7 cell. Each value is expressed as mean ± SE (n=3). Each NSAIDs group and DEX group showed a significantly lower value than the CTL group (not show mark). #Statistically significant differences were seen when compared to the each NSAIDbulk (P<0.05). *There were statistically significant differences when compared with the IMCnano group (P<0.05).
Discussion
In this study, there was a difference between NSAIDbulkand NSAIDnano in all results. NSAIDs are reversible inhibitors of cyclooxygenase. They have excellent anti-inflammatory and analgesic effects and are widely used in the treatment of inflammatory diseases, such as chronic rheumatoid arthritis, and chronic musculoskeletal pain, such as low back pain and arthralgia, and for postoperative analgesia [14,15].
Drug systems using nanoparticles are expected as a strategy to increase drug bioavailability. Because nanoparticles improve the bioavailability and reduce the required doses of orally administered NSAIDs, their use in the treatment of patients with inflammatory diseases is expected to increase. In the present study, we prepared 3 types of NSAIDnano and investigated the influence of differences in the particle size on the anti-inflammatory effects of these drugs on mouse macrophages, or RAW cells. The NSAIDs used in this study are also under consideration for nanoization in other reports. Our nanozoning method does not use special equipment. It is carried out using an easyto- obtain rotating and revolving crusher (NP-100). Also, no organic solvent or stabilizer is added. In these respects, it is different from other reports and we believe it is an advantage of our method. Regarding the stability of NSAIDnano, it was confirmed the stability of the nanoparticles for 14 days (not show data) although it is not shown because it is being posted to another publication. In this study, NSAIDs showed dosedependent cytotoxicity on the LPS-stimulated macrophage cell line (RAW 264.7); no cytotoxicity was observed at low concentrations.
In addition, the NSAIDnano reduced the viability of the macrophages to a significantly greater degree than unprocessed NSAIDbulk at high concentrations. A macrophage receptor with a collagenous structure expressed on the macrophage cell surface is reported to be involved in the phagocytosis of unopsonized particles [16], and also in the cellular binding and incorporation of nanoparticles [17]. Intracellular nanoparticles tend to accumulate in the mitochondria and are involved in the generation of reactive oxygen species and in the reduction of antioxidant activity, thereby causing cell damage [18-21]. Many NSAIDnano at high concentrations were considered to be incorporated into the macrophages and to cause cell damage, thereby decreasing the viability of the cells. Therefore, the concentration of 20 μg/mL, at which there were no differences in the effects on the nanoparticles on the cell viability, was used to evaluate anti-inflammatory effects. Thus, the antiinflammatory effects of the NSAIDs on the mouse macrophages in this study were unlikely to be affected by their cytotoxicity.
Evaluation of the anti-inflammatory effects of NSAIDnano on LPSinduced acute inflammation in the mouse macrophages revealed that the NSAIDs suppressed the production of NO and inflammatory cytokines (TNF-α and IL-6) by the macrophages, and that the degree of the effect differed depending on the particle size of the NSAIDnano. The NSAIDnano suppressed the production of NO and inflammatory cytokines to a significantly greater degree than the unprocessed NSAIDbulk. Among the NSAID nanoparticles, IMCnano was the most effective at suppressing the production of NO, TNF-α and IL-6 by the macrophages. In addition, the NSAIDnano also suppressed the production of prostaglandin E2 to a greater degree than the unprocessed NSAIDbulk. Among the NSAID nanoparticles, IMCnano was the most effective at suppressing the production of prostaglandin E2. These results suggest that IMCnano exerted the strongest antiinflammatory effects. As NSAIDnano suppress the production of NO and other inflammatory cytokines to a significantly greater degree than unprocessed NSAIDbulk, they can be considered as having stronger anti-inflammatory effects. iNOS is induced by inflammation and stress [22,23]. During inflammation, a large amount of iNOS is expressed, with excessive production of NO, thereby damaging cells [24,25]. NO produced in the body has a variety of physiological activities, such as the regulation of vasorelaxation [26], neurotransmission [27] and infection [28]/inflammatory reactions [29]. On the other hand, NO has also been reported to be involved in carcinogenesis through its effects on DNA damage, angiogenesis and immunosuppression [29-32]. NSAIDnano used in the present study suppressed the production of NO and PG to a much stronger degree than unprocessed NSAIDbulk, thereby exerting strong anti-inflammatory effects. however, the stronger suppression of NO and PG, which protect the gastrointestinal mucosa, production by NSAIDnano than by unprocessed NSAIDs suggests that NSAIDnano may exacerbate the adverse reactions to NSAIDbulk, especially gastrointestinal and renal disorders. NSAIDnano used for this study showed a strong antiinflammatory effect by suppressing NO and PG production from the NSAIDbulk. This suggests that the NSAIDnano preparation may be a raw material for new drugs. In addition, it was suggested that NSAIDnano preparation could reduce the concentration of the drug. These facts can be considered to lead to miniaturization of preparations and suppression of medical expenses if the content of the drug is reduced. However, it is considered that the formulation containing NSAIDs nanoparticles may change the in vivo dynamics and safety, and it is considered that the same evaluation as new drugs is necessary for use. In the future, detailed animal studies are required on the influence of NSAID nanoparticles on the gastrointestinal tract and kidney, the optimal route of administration of these particles, etc.
Conflict of Interest
The authors declare that they have no conflict of interest to disclose.
Acknowledgments
The authors would like to thank Department of Pharmacy, Kochi Medical School Hospital for technical assistance with the experiments.
23272
References
- Kundu JK, Surh YJ (2008) Inflammation: gearing the journey to cancer. Mutat Res 659: 15-30.
- Kang HJ, Jeong JS, Park NJ, Go GB, Kim SO, et al. (2017) An ethanol extract of Aster yomena (Kitam.) Honda inhibits lipopolysaccharide-induced inflammatory responses in murine RAW 264.7 macrophages. Biosci Trends 11: 85-94.
- Watanabe M, Hayasaki H, Yurugi Y (2017) Mechanisms of pain and pain relief. JAHS 8: 50-63.
- Eguchi H, Fujiwara N, Ookawara T, Suzuki K, Taniguchi N (2009) Oxidative stress and health. J Anal BioSci 32: 247-256.
- Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373-376.
- Singer II, Kawka DW, Scott S, Weidner JR, Mumford RA, et al. (1996) Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111: 871-885.
- Peng W, Ma YY, Zhang K, Zhou AY, Zhang Y, et al. (2016) Synthesis and biological evaluation of novel resveratrol-NSAID derivatives as anti- inflammatory agents. Chem Pharm Bull 64: 609-615.
- Chao SH, Wu AB, Lee CJ, Chen FA, Wang CC (2005) Anti-inflammatory effects of indomethacin's methyl ester derivative and induction of apoptosis in HL-60 Cells. Biol Pharm Bull 28: 2206-2210.
- Wallace JL, McKnight W, Reuter BK, Vergnolle N (2000) NSAID-induced gastric damage in rats: Requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology 119: 706-714.
- Cryer B, Feldman M (1998) Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med 104: 413-421.
- Takeda S, Okazaki H, Ikeda E, Abe S, Yoshioka Y, et al. (2014) Down-regulation of cyclooxygenase-2 (COX-2) by cannabidiolic acid in human breast cancer cells. J Toxicol Sci 39: 711-716.
- Hirose A, Nishimura T, Kanno J (2009) Research strategy for evaluation methods of the manufactured nanomaterials in NIHS and importance of the chronic health effects studies. Bull Natl Inst Health Sci 127: 15-25.
- Heinemann M, Schäfer HG (2009) Guidance for handling and use of nanomaterials at the workplace. Hum Exp Toxicol 28: 407-411.
- Süleyman H, Demircan B, Karagöz Y (2007) Anti-inflammatory and side effects of cyclooxygenase inhibitors. Pharmacol Rep 59: 247-258.
- Vane JR, Botting RM (1998) Anti-inflammatory drugs and their mechanism of action. Inflamm Res 47: 78-87.
- Kobzik L (1995) Lung macrophage uptake of unopsonized environmental particulates. Role of scavenger-type receptors. J Immunol 155: 367-376.
- Kanno S, Furuyama A, Hirano S (2007) A murine scavenger receptor MARCO recognizes polystyrene nanoparticles. Toxicol Sci 97: 398-406.
- Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, et al. (2004) Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect 112: 1347-1358.
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, et al. (2003) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111: 455-460.
- Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, et al. (2002) Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun 294: 116-119.
- Gopinath PG, Gopinath G, Kumar TCA (1978) Target site of intranasally sprayed substances and their transport across the nasal mucosa: A new insight into the intranasal route of drug delivery. Curr Ther Res 23: 596-607.
- Yamaguchi K, Saito H, Oro S, Tatebe S, Ikeguchi M, et al. (2005) Expression of inducible nitric oxide synthase is significantly correlated with expression of vascular endothelial growth factor and dendritic cell infiltration in patients with advanced gastric carcinoma. Oncology 68: 471-478.
- Osanai Y, Kanno S, Sasaki T, Hiratsuka M, Ishikawa M (2008) Inhibition of Lipopolysaccharide-induced Expression of Inducible Nitric Oxide Synthase by Caffeic Acid Undecyl Ester in RAW 264.7 Macrophages. Journal of Tohoku Pharmaceutical University 55: 99-109.
- Shimokawa H (1997) Recent Advances in Research on Nitric Oxide. Saishin-Igaku 52: 909-919.
- Tutsui M, Ueno S, Toyohira Y, Yanagihara N (2002) Structure and function of nitric oxide synthases. Protein, Nucleic Acid and Enzyme 47: 2024-2031.
- Radomski MW, Palmer RM, Moncada S (1987) The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun 148: 1482-1489.
- Vincent SR (2010) Nitric oxide neurons and neurotransmission. Prog Neurobiol 90: 246-255.
- Akaike T (2001) NO in Host Defense and Microbial Pathogenesis. Nihon Saikingaku Zasshi 56: 503-511.
- Akaike T (2015) Host defense and oxidative stress signaling in bacterial infection. Nihon Saikingaku Zasshi 70: 339-349.
- Chen F, Oikawa S, Hiraku Y, Murata M, Yamashita N, et al. (1998) Metal-mediated oxidative DNA damage induced by nitro-2-aminophenols. Cancer Lett 126: 67-74.
- Ozawa K, Tsumoto H, Wei W, Tang CH, Komatsubara AT, et al. (2012) Proteomic analysis of the role of S-nitrosoglutathione reductase in lipopolysaccharide-challenged mice. Proteomics 12: 2024-2035.
- Tazawa H, Kawaguchi T, Kobayashi T, Kuramitsu Y, Wada S, et al. (2013) Chronic inflammation-derived nitric oxide causes conversion of human colonic adenoma cells into adenocarcinoma cells. Exp Cell Res 319: 2835-2844.









