Keywords
Polymers, Release Kinetics, Transdermal patch, Ethyl cellulose, HPMC, Polyvinyl pyrollidone
Introduction
Modern transdermal drug delivery systems are products of basic pharmaceutical research that took place during the last third of the 20th century. The first drug delivered through the skin was dimethyl sulfoxide in 1900 and nitroglycerine ointment was introduced for the management of angina in 1954.[1] However, these attempts lacked the proper scientific foundation and were short-lived. The evolution of transdermal drug delivery systems that had a meaningful impact on the practice of medicine is intimately linked to the name of Dr. Alejandro Zaffaroni, a US scientist who, in the late 1960s, founded the Alza Corporation.[2] Transdermal drug delivery system are designed for controlled drug delivery through skin surface into blood circulation maintaining consistent efficacy and reducing dose of the drug and its related side effects.[3] The US Food and Drug Administration (FDA) approved the first transdermal products in 1981. Transdermal delivery provides increase in the therapeutic value of many drugs by avoiding specific problems associated with the drug e.g., gastro-intestinal irritation, low absorption, decomposition due to hepatic “ first pass” effect, formation of metabolite that causes side effects, short half life necessitating frequent dosing etc.[4,5] Verapamil hydrochloride was the first amongst the calcium-channel blockers (CCBs). It has been used since 1962 in Europe, then in Japan for its antiarrhythmic and coronary vasodilator effects. [6,7] Verapamil hydrochloride is the drug of choice for controlled delivery as the drug is reported to have a terminal plasma half life of 2 to 8 hours following single oral dose or intravenous administration but is subject to very considerable first-pass metabolism in the liver and the bioavailability is only about 20%.[8,9,10] Verapamil hydrochloride transdermal patches were prepared using different polymer combinations namely ethyl cellulose with polyvinyl pyrrolidone K30 and with hydroxy propyl methyl cellulose K4M using solvent evaporation technique. Polyvinyl alcohol (4% w/v) was used to prepare the backing membrane and Dibutyl phthalate (30% w/w) was used as plasticizer. The prepared patches were examined in respect to several physicochemical properties thickness, percent moisture content, percent moisture absorption, percent flatness, tensile strength, weight variation to satisfy the suitable physicochemical criteria for transdermal patch. For all the formulations, invitro release and skin permeation of the drug with and without incorporation of DMSO (15% w/w) as penetration enhancer, through abdominal skin of albino rat were studied using Keshary-Chien diffusion cell.[11,12]
Material and Methods
Ethyl cellulose, Dimethyl sulphoxide, Polyvinyl alcohol were procured from S.D. Fine Chemicals Ltd. Mumbai. Polyvinyl pyrrolidone K30 was procured from Thomas Baker, Mumbai. Hydroxy propyl methyl cellulose K4M was procured from Loba Chemicals; Mumbai. Verapamil hydrochloride was obtained as gift sample from Torrent Pharmaceuticals Ltd; Ahmedabad. Aluminium foil used were purchased from S.R. Industries; New Delhi. All other materials used in the study were of analytical grade.
Preparation Of The Patches
Transdermal patches of verapamil hydrochloride were prepared using different combinations of EC with PVP K30 and EC with HPMC K4M by solvent evaporation technique[13] in cylindrical both side opened glass molds. The bottom of the mold was wrapped with aluminium foil on which the backing membrane was cast by pouring 4% (w/v) PVA solution followed by drying at 60? C for 6 hours. The polymers were weighed in requisite ratio (Table-1) and they were then dissolved in respective solvents. Dibutyl phthalate 30% (w/w) of total polymer composition was used as a plasticizer. The drug was added 20% (w/w) of the total weight of polymer, in the homogeneous dispersion, by slow stirring with a magnetic stirrer. The uniform dispersion (2 ml each) was casted on the PVA backing membrane casted earlier and dried at 40? C for 6 hours. After drying patches were removed from the mold, wrapped with aluminium foil and kept in desiccators until they were used for further study.
Evaluation Of Polymeric Transdermal Patches
Uniformity of thickness
The thickness of the patch at five different points was measured with micrometer (Mitutoyo) and the average of five readings with the standard deviation was calculated[14]. The procedure was followed for all the formulation batches.
Percent moisture content (% MC)
The patches were weighed individually and kept in a desiccator containing 10 gm of calcium chloride as desiccant at 37°C for 24 hour. The patches were weighed again and again individually until it showed a constant weight. The final weight was noted when there was no further change in the weight of individual patch. The percentage of moisture content was calculated[15] as a difference between initial and final weight with respect to final weight.
% Moisture content = [(x-y)/y] * 100, Where, x = initial weight, y = finial weight
The result of moisture content studies for different formulations are shown in Fig 1. Each result is mean of three replicates.
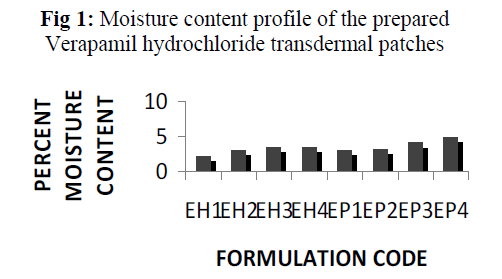
Fig 1: Moisture content profile of the prepared Verapamil hydrochloride transdermal patches
Percentage moisture uptake (% MU):
The patches were weighed accurately and placed in a desiccator where a humidity condition of 75 % RH was maintained by using saturated solution of sodium chloride. The patches were taken out periodically and weighed for a period of 72hours. The percentage of moisture uptake was calculated[15] as difference between final and initial weight of the patch with respect to initial weight.
% Moisture uptake = [(y-x)/x] * 100, Where, x = initial weight, y = finial weight.
The result of moisture content studies for different formulations are shown in Fig 2. Each result is mean of three replicates.
Percent flatness study:
Longitudinal strips were cut out from each transdermal patch, one from the centre and two from the either side. The length of each strip was measured and the variation in the length because of non-uniform in flatness was measured by determining % constriction, considering 0 % constriction is equivalent to 100 % flatness.[15] % constriction = [(l1 – l2)/l2] *100, Where, l1 = initial length of each strip,l2 = final length of each strip.
Tensile strength:
The tensile strength measurement was made using an instrument assembled in the laboratory and following the method used by Sadhna et al.[16] The films were fixed individually to the assembly, the required weights to break the films were noted.
Weight variation study:
This test provides a means for measuring uniformity in terms of the weight within a batch as well as batch to batch. The weight of each patch was taken using single pan balance with sensitivity of 0.001 mg. [17,18]
Invitro permeation studies using dialysis membrane: [19]
Invitro permeation studies were carried out using modified Keshary – Chien diffusion cell. The dialysis sac was previously soaked for 24 hours in distilled water. The patches were adhered to the barrier membrane (dialysis membrane) and the sac is tied firmly to the donor compartment of the Keshary – Chien diffusion cell, the receptor compartment of which is filled with 100 ml distilled water. The donor compartment is lowered to the receptor compartment in such a way that the dialysis sac just touches the media of the receptor compartment. The total setup was placed on a thermostatically controlled magnetic stirrer set at 37 ± 2°C. The content of the diffusion cell was stirred using a teflon coated bead at a constant speed (100 rpm). Samples were withdrawn (1 ml) at predetermined time intervals and replaced with same amount of distilled water to maintain the sink condition. After suitable dilution, the samples were analyzed for drug content using UV spectrophotometer at λmax 229.5 nm. The permeation study was carried out for 24 hours.
Invitro skin permeation study using albino rat skin: [19, 20]
Invitro skin permeation study was performed taking albino rat skin. Young albino rat weighed between (200 gm- 250 gm) were taken and sacrificed by excess chloroform inhalation. The abdominal hairs were removed with marketed hair removers. The abdominal skin was carefully separated from the body, with the dermis part remaining intact. Subcutaneous tissues were surgically removed. The inner part of the skin was washed with distilled water thoroughly to separate the adhering fat. The skin, so obtained, was examined microscopically for the presence of any possible damage. The full thickness skin thus obtained was kept in normal saline solution and stored at 4 ± 1°C until used for the experiment.
The drug permeation from the transdermal patches through the skin was determined using modified Keshary – Chien diffusion cell. The contents of the donor and receptor compartments were separated by placing the excised skin in between two compartments. The skin was mounted in such a way that the stratum corneum side of the skin continuously remained in an intimate contact with the transdermal patch in the donor compartment. The receptor compartment contained 100ml distilled water at 37 ± 2°C. The content of the diffusion cell was stirred using a teflon coated bead at a constant speed (100 rpm). Samples were withdrawn (1 ml) at predetermined time intervals and replaced with same amount of distilled water to maintain the sink condition. After suitable dilution the samples were analyzed for drug content using UV spectrophotometer at λmax 229.5 nm. The permeation study was carried out for 24 hours.
Results
In the present study transdermal patches of Verapamil hydrochloride were prepared as monolithic matrices by solvent casting technique employing glass moulds of known diameter. Transdermal patches were prepared using various polymers like ethyl cellulose with HPMC K4M in different combinations and ethyl cellulose with PVP K30 in different combinations (Table 1).
| Sl . No. |
Formulation code |
Ratio of EC: HPMC |
Ratio of EC: PVP |
Total weight of Polymer |
Solvent |
Plasticizer (% w/w) of total polymer |
Drug (% w/w) of total polymer |
| Ethanol |
Chloroform |
| 1. |
EH1 |
8:1 |
------- |
500 mg |
10 ml |
------- |
30 |
20 |
| 2. |
EH2 |
6:1 |
------- |
500 mg |
10 ml |
------- |
30 |
20 |
| 3. |
EH3 |
4:1 |
------- |
500 mg |
10 ml |
------- |
30 |
20 |
| 4. |
EH4 |
2:1 |
------- |
500 mg |
10 ml |
------- |
30 |
20 |
| 5. |
EP1 |
------- |
8:1 |
500 mg |
------- |
10 ml |
30 |
20 |
| 6. |
EP2 |
------- |
6:1 |
500 mg |
------- |
10 ml |
30 |
20 |
| 7. |
EP3 |
------- |
4:1 |
500 mg |
------- |
10 ml |
30 |
20 |
| 8. |
EP4 |
------- |
2:1 |
500 mg |
------- |
10 ml |
30 |
20 |
Table-1: Formulation chart of Verapamil hydrochloride transdermal patch.
The different physicochemical characteristics of different patches along with there release characteristics were studied. The thickness of the patches prepared with ethyl cellulose and HPMC K4M were found in between 0.00168 cm to 0.00206 cm and thickness of the patches prepared with ethyl cellulose and PVP K30 were found between 0.00174cm to 0.00200 cm (Table 2).
| Formulation Code |
Mean thickness
± S.D.**
(cm) n=5 |
Percent flatness n=3 |
Tensile strength(gm/cm2) |
Weight variation(gm)
± S.D.
n=3 |
| EH1 |
0.00198 ± 0.00083666 |
100 |
321 |
0.140 ± 0.0030 |
| EH2 |
0.00206 ± 0.00017310 |
100 |
318 |
0.141± 0 .0010 |
| EH3 |
0.00168 ± 0.00010954 |
100 |
309 |
0.122 ± 0.0010 |
| EH4 |
0.00174 ± 0.00089442 |
100 |
299 |
0.125 ± 0.0030 |
| EP1 |
0.00200 ± 0.00070710 |
100 |
267 |
0.143 ±0.0035 |
| EP2 |
0.00198 ± 0.00083666 |
100 |
259 |
0.134 ± 0.0032 |
| EP3 |
0.00176 ± 0.00054772 |
100 |
242 |
0.131 ± 0.0058 |
| EP4 |
0.00174 ± 0.00054772 |
100 |
239 |
0.134 ± 0.0025 |
** ± S.D. is the standard deviation, n= no. of repeated experiment.
Table 2: Thickness, percent flatness, tensile strength weight variation profile of the prepared Verapamil hydrochloride transdermal patches.
The percent moisture content(Fig 1) and the percent moisture uptake (Fig 2) of the patches showed that the moisture content and moisture uptake increases gradually with the increase of hydrophilic polymer concentration for different formulations containing HPMC K4M and PVP K30 with ethyl cellulose. Formulation EH1 and EP1 were found to be the best amongst all the formulations prepared of ethyl cellulose with combination of HPMC K4M and PVP K30 in this respect. The patches were found flat and weight variation among each of the formulation were found within acceptable range.
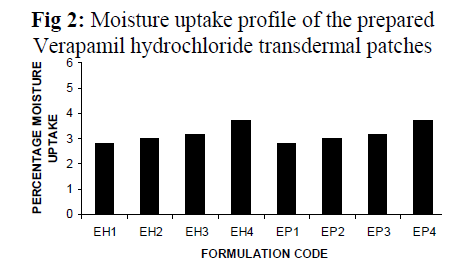
Fig 2: Moisture uptake profile of the prepared Verapamil hydrochloride transdermal patches.
The invitro permeation of drug from the prepared patches were carried out in modified Keshary-Chein diffusion cell through dialysis membrane using 100 ml distilled water as diffusion media for a period of 24 hours. The graphical representation of Cumulative % drug release as a function of time is shown in (Fig 3 and Fig 4). Formulation like EH3, EH4, EP3 and EP4 were chosen for further invitro skin permeation study with and without DMSO, through albino rat skin as they showed a greater release of drug compared to formulation EH1,EH2, EP1 and EP2. Comparative graphs were plotted after skin permeation study of EH3, EH4, EP3 and EP4. (Fig 5). An enhancement in drug permeation was observed with the incorporation of DMSO (Fig 6). The data from invitro skin permeation study of different formulation were fitted to various kinetic models like Zero order kinetic model and Higuchi kinetic model and the coefficient of regression were computed (Table 3 and table 4).
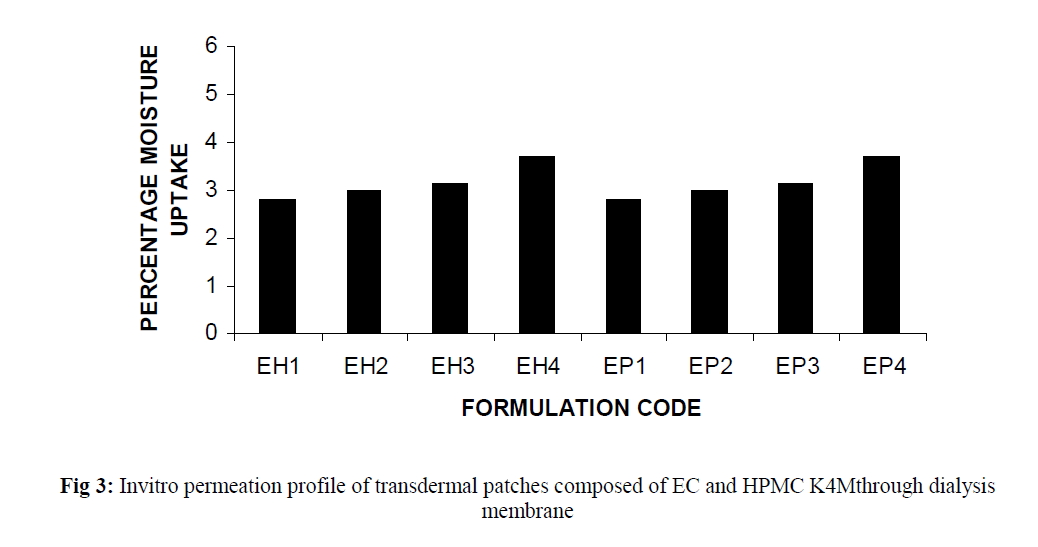
Fig 3:Invitro permeation profile of transdermal patches composed of EC and HPMC K4Mthrough dialysis membrane.
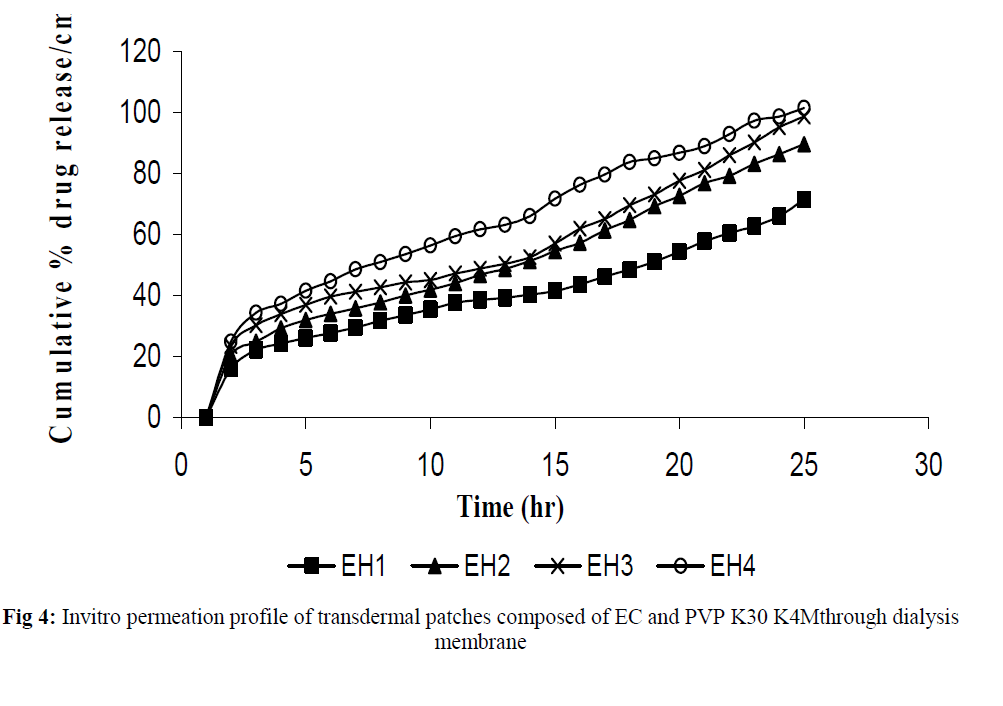
Fig 4:Invitro permeation profile of transdermal patches composed of EC and PVP K30 K4Mthrough dialysis membrane
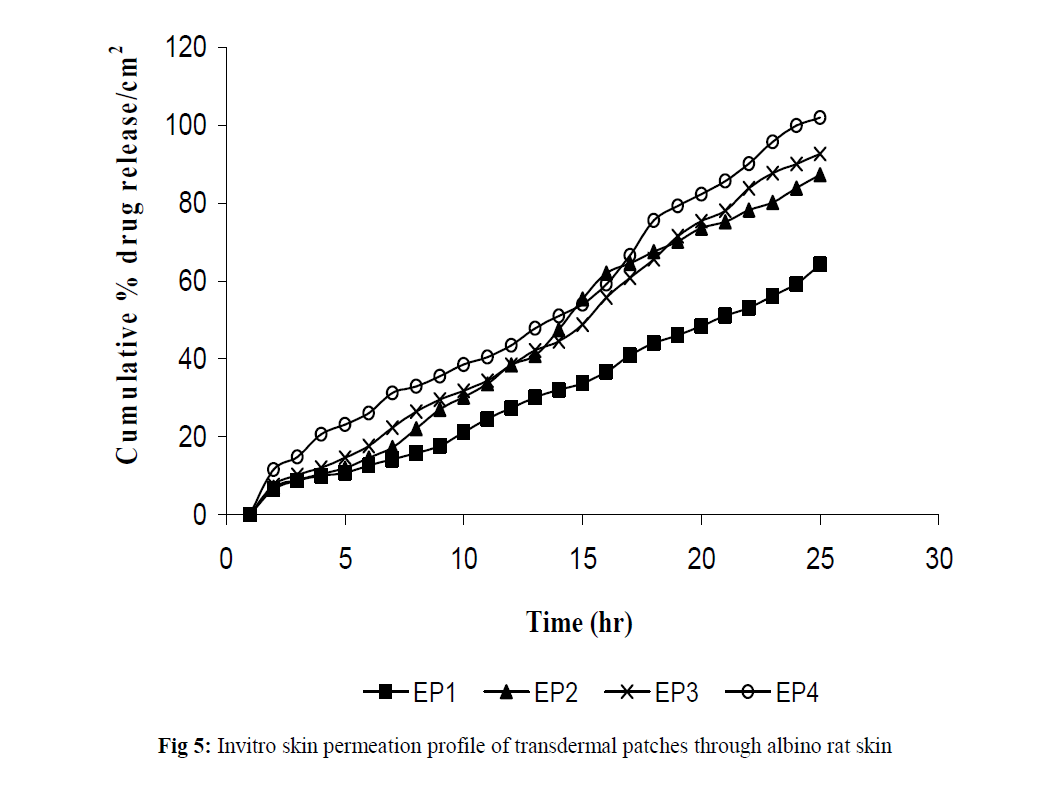
Fig 5:Invitro skin permeation profile of transdermal patches through albino rat skin.
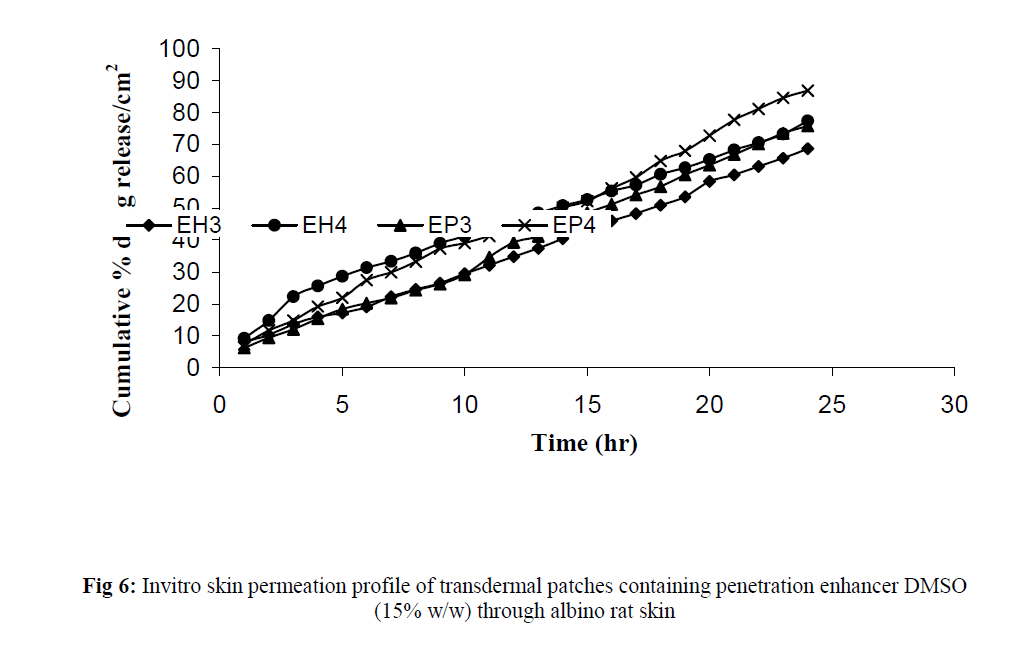
Fig 6:Invitro skin permeation profile of transdermal patches containing penetration enhancer DMSO (15% w/w) through albino rat skin.
| Sl no |
Formulation code |
Ratio of EC:HPMC K4M |
%Cumulative permeated Vs Time
(Zero order model) Correlation coefficient (r2) |
%Cumulative permeated Vs
√T
(Higuchi model) Correlation coefficient (r2) |
| 1 |
EH3 |
4:1 |
0.9886 |
0.9880 |
| 2 |
EH3 with
DMSO |
4:1 |
0.9932 |
0.9601 |
| 3 |
EH4 |
2:1 |
0.9973 |
0.9534 |
| 4 |
EH4 with
DMSO |
2:1 |
0.9959 |
0.9823 |
Table 3: Incorporated invitro skin permeation results of transdermal patch formulations composed of EC and HPMC K4M with and without penetration enhancer (15%DMSO) into two kinetic models.
| Sl no |
Formulation code |
Ratio of EC:PVP K30 |
%Cumulative permeated Vs Time
(Zero order model) Correlation coefficient (r2) |
%Cumulative permeated Vs
√T
(Higuchi model) Correlation coefficient (r2) |
| 1 |
EP3 |
4:1 |
0.9963 |
0.9544 |
| 2 |
EP3 with DMSO |
4:1 |
0.9934 |
0.9851 |
| 3 |
EP4 |
2:1 |
0.9963 |
0.9544 |
| 4 |
EP4 with DMSO |
2:1 |
0.9944 |
0.9842 |
Table 4: Incorporated invitro skin permeation results of transdermal patch formulations composed of EC and PVP K30 with and without penetration enhancer (15%DMSO) into two kinetic models.
Fig 7:Scanning electron Microphotographs of TDDS of Verapamil hydrochloride.
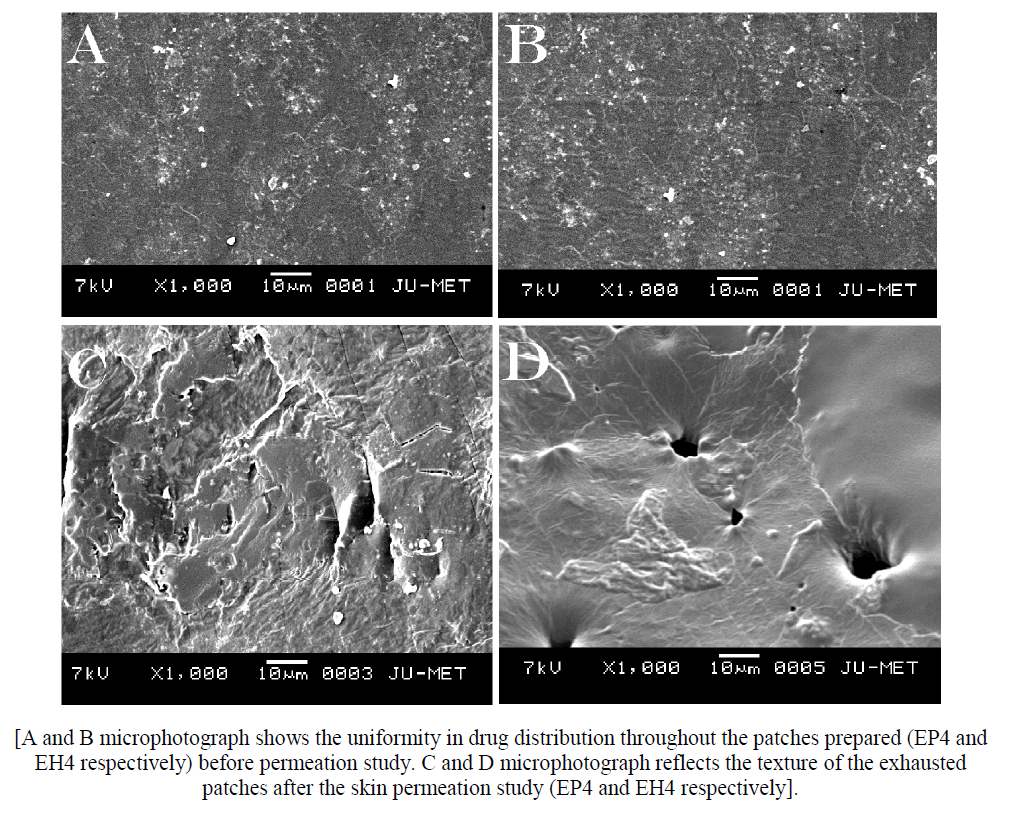
Fig 8:[A and B microphotograph shows the uniformity in drug distribution throughout the patches prepared (EP4 and EH4 respectively) before permeation study. C and D microphotograph reflects the texture of the exhausted patches after the skin permeation study (EP4 and EH4 respectively]./p>
Discussion
Transdermal patches were prepared by solvent casting technique. The patches were clear, transparent and flexible which shows reliability of the process development of films. The different physicochemical characteristics of different patches along with there release characteristics were studied. Low standard deviation values in the patch thickness measurement ensures uniformity of the patches prepared by solvent casting technique.the percent moisture content and percent moisture uptake study reveals the pattern of the patches in moisture content and moisture uptake phenomena. A little moisture content of the formulations help them not to become completely dried and brittle. Again low moisture uptake value protects microbial contamination of the formulations. The uniformity in flatness of the prepared patches indicates that the formulation by solvent evaporation technique is reproducible and the formulations can maintain satisfactory surface smoothness. It was observed from the tensile strength measurement study that with the increase of HPMC K4M and PVP K30 concentration, the tensile strength of the patches gradually decreased. All the patches prepared showed uniformity in weight. The graphical representation of Cumulative % drug release as a function of time showed that the patches releases drug over an extended period of 24 hrs. Comparative plots of drug release reveals that the drug permeation is lesser through the skin compared to permeation through dialysis membrane. DMSO significantly increased the drug permeation through transdermal patches. Plots of % Cumulative drug permeated/cm2 as a function of time was found to be linear for all the formulations showing straightness of the plot between % Cumulative drugs permeated versus time. The drug release from the transdermal patch formulations may obey zero order release kinetics.
Conclusion
From the above study it can be concluded that a suitable blend of polymers can optimized the drug release from transdermal patches for an extended period of time to improve patient compliance.
5373
References
- Scheuplein RJ. Mechanism of percutaneous absorption: Routes of penetration and the influence of solubility. J Invest Dermatol 1965; 45: 334-339.
- Zaffaroni A. Overview and evolution of therapeutic systems. Ann. NY Acad Sci 1991; 618: 405ñ421.
- Funke AP, Gunther C, Muller RH, Lipp R. Development of matrix patches for transdermal delivery of a highly lipophilic antiestrogen. Drug Develop Ind Pharm 2003; 29: 785-793.
- Chien YW, Robinson JR, Lee VH. Transdermal Therapeutic System, Novel Drug Delivery: Fundamentals and Application. Marcel Dekker Inc., NewYork, 1982, pp 524.
- Misra AN, Jain NK. Transdermal Drug Delivery: Controlled and Novel Drug Delivery. New Delhi, CBS Publisher and Distributors, 1997, pp 100- 129.
- Durand PG, Lehot JJ. Calcium-channel blockers and anaesthesia. Can J Anaesth 1991; 38: 75 ñ 89.
- Alastair JJ. Calcium antagonist drugs. The New England J Med 1999; 341: 1447-1457.
- Sweetman SC. Martindale: The Complete Drug Reference. Pharmaceutical Press, 2005, pp 1019.
- Moffat AC, Osselton MD, Widdop B. Clarkeís Analysis of Drugs and Poisons. Pharmaceutical Press, 2004, pp 1695.
- Indian Pharmacopoeia, Vol. II, Government of India. The Controller of Publication, New Delhi, 1996, pp 797-799.
- Manvi FV, Dandagi PM, Gadad AP, Mastiholimath VS, Jagadeesh T. Formulation of a transdermal drug delivery system of ketotifen fumarate. Indian J Pharm Sci 2003; 65: 239-243.
- Bharkatia M, Nema RK, Gupta GD, Gaud RS. Designing and evaluation of propranolol hydrochloride transdermal patch. The Pharma Review 2005; June: 113-116.
- Das MK, Bhattacharya A, Ghosal SK. Transdermal delivery of trazodone hydrochloride from acrylic films prepared from aqueous latex. Indian J Pharm Sci 2006; 68: 41-46.
- Arora P, Mukherjee B. Design, development, physicochemical, invitro and invivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J Pharm Sci 2002; 91: 2076-2089.
- Gupta R, Mukherjee B. Development and invitro evaluation of diltiazem hydrochloride transdermal patches based on povidone ñ ethylcellulose matrices, Drug Develop. Ind. Pharm. 2003; 29: 1-7.
- Gupta SP, Jain SK. Effective and controlled transdermal delivery of metoprolol tartarate. Indian J. Pharm. Sci. 2005; 67: 346-350.
- Panigrahi L, Pattnaik S, Ghosal S.K. Permeation kinetics of diclofenac sodium from pseudolatex transdermal formulations through lipidized and
- Saxena M, Mutalik S, Reddy MS. Formulation and evaluation of transdermal patches of metoclopramide hydrochloride. Indian Drugs 2006; 43: 740-745.
- Zhao H, Park DW, Kim SK, Lee CH, Kim DD. The effect of pressure-sensitive adhesive and solubilizers on the skin permeation of testosterone from a matrix type transdermal delivery system. Drug Develop. Ind. Pharm. 2002; 28: 1125-1131.
- Chakraborty P, Dey BK, Bahadur S, Thakkar S, Das S. Design, Development, Physicochemical and In Vitro Evaluation of Transdermal Patches Containing Verapamil Hydrochloride in Ethyl Cellulose - Povidone Matrices. Research J. Pharm. and Tech. 2009; 2: 168-172.














