Key words
|
| |
| Ethylene glycol, Rotula aquatica, Boraginaceae, Urolithiasis |
| |
Introduction
|
| |
| Urolithiasis (renal stone formation) is a recurrent disorder predominant in males. The present day medical management of urolithiasis is either costly or not without side effects. Hence, the search for antilithiatic drugs from natural sources has assumed greater importance. Many Indian plants have been quoted to be useful as antilithiatic agents. They are effective with fewer side effects and are also inexpensive. Hence; the Indian plants are constantly being evaluated for possible antilithiatic effects in a systematic manner.1 One such plant is Rotula aquatica (Boraginaceae), which is used as antiinflammatory2, diuretic, laxative, in hemorrhoids, renal and vesical calculi, diabetes and venereal diseases.3 The plant contains Baunerol, steroid, alkaloid and rhabdiol. The medicinal values of plant lie in their component Phytochemical such as alkaloids, flavonoids, phenolic compounds and other nutrients like as amino acid and proteins.4 The traditional Indian medicine, Ayurveda suggests this plant to be antilithiatic, but scientific data supporting this statement is still lacking. Hence this study was undertaken. |
| |
Materials and Methods
|
| |
|
Animals
|
| |
| Male albino Wistar rats (180-200g) were obtained from HAU (Hisar, India).They were housed in wellventilated cages (3 to 4 per cage), maintained at 25±2°C under 12-h dark/light cycle. They were fed gram diet and had free access to water. The animals were maintained in these conditions for 1 week before the experimental session. Our institutional animal ethical committee (IAEC) approved this study. |
| |
|
Plant material
|
| |
| Roots of Rotula aquatica were collected from the medicinal farm at Salasar (Rajasthan, India) and identified by the Department of botany, The American College (Madurai, India) and a voucher specimen (MAC 16673) was deposited in our laboratory. The leaves was shade dried and coarsely powdered in such a way that it passed through sieve no-20 and was retained on sieve no-40. About 1Kg of the dry powder was soxhlet- extracted with 70% ethyl alcohol, in 1:5 w/v ratios for 72 h. The yield was 20 g. The extract was dried in vacuum and suspended in water before use. |
| |
|
Antilithiatic Study
|
| |
| Ethylene glycol induced lithiasis was used to assess the antilithiatic activity in albino rats.5 The acclimatized animals were divided into 4 groups, from Group I to Group IV, each with 6 rats and urolithiasis (stone) was induced in all the groups except Group-I as it was reserved as “Normal control”. Group II, animals received 1% ethylene glycol in drinking water ad libitum for 28 days and served as the lithiatic control. The G-III group animals received 1% ethylene glycol in drinking water ad libitum; along with alcoholic extract of Rotula aquatica (200mg/kg body weight) 3 and G-IV group animals received 1% ethylene glycol in drinking water ad libitum, along with Cystone (100 mg/kg body weight) orally 28 days. |
| |
|
Collection and analysis of urine
|
| |
| Animals were kept in separate metabolic cages and urine samples of 24 h were collected on day 28. A drop of concentrated hydrochloric acid was added to the urine before being stored at 4°C. Urine was analyzed for calcium, phosphate, oxalate, protein, creatinine, Magnesium and uric acid content. (6, 7, 8 9) |
| |
|
Serum analysis
|
| |
| After the experimental period, blood was collected from the retro-orbital under anesthetic conditions and the animals were sacrificed by cervical decapitation. Serum was separated by centrifugation at 10,000 rpm for 10 min and analyzed for calcium, phosphate, oxalate, creatinine, Magnesium and uric acid content. |
| |
Histopathological Studies
|
| |
| Kidney samples were weighed and fixed rapidly with 10% neutralized formalin (pH 7.4). Sections of kidney fixed in paraffin were prepared and stained with hematoxylin and eosin and observed for histopathological changes. |
| |
|
Statistical analysis
|
| |
| The results are expressed as Mean ± SEM. Data was evaluated using ANOVA; Single factor test followed by Newman – Keuls multiple range tests. Probability values less than (p<0.05) were considered significant. |
| |
Results
|
| |
|
Urinary and Serum Data
|
| |
| The calcium excretion was increased significantly on the 28th day in the ethylene glycol treated rats (G-II) compared with the normal control rats (G-I). However the calcium excretion was reduced in the extract treated group (G-III) but calcium excretion was reduced significantly in the Cystone treat group (G-IV), though normal values were not reached. Urinary data are shown in Table 1 and serum data are shown in table 2. |
| |
| Likewise, ethylene glycol treatment increased phosphate, oxalate, protein, uric acid and creatinine excretion significantly in the lithiatic control group (G-II). However treatment with the extract were reduced but significantly with Cystone treatment (GIV), though normal values were not reached. The results are shown in Table 1 and Table 2. |
| |
| The urinary magnesium on the 28th day was reduced after treatment with ethylene glycol in lithiatic control (G-II). Simultaneous administration of the extract (G-III) elevated the reduced magnesium level but significantly elevated in Cystone treatment (GIV) when compared with the lithiatic control group (G-II). The result is shown in Table 1and Table 2. |
| |
|
Histopathological Data
|
| |
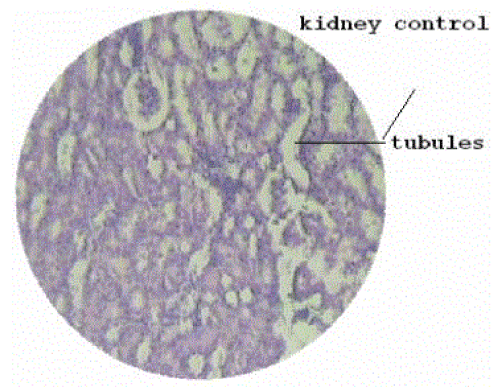
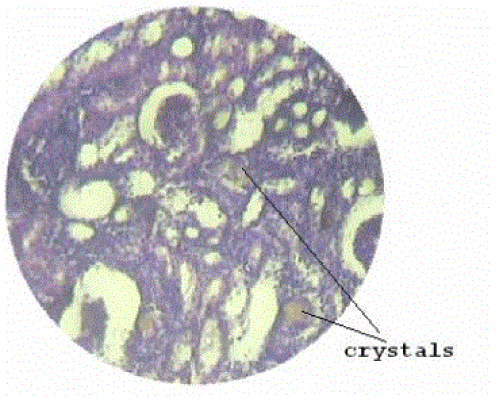
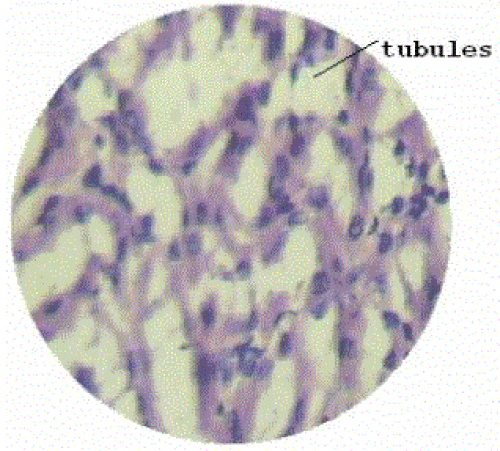
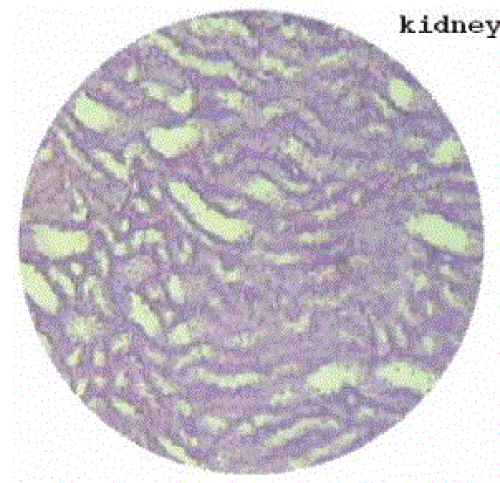
| Section of kidney from animals treated with ethylene glycol showed that deposition of micro- crystals (Figure-2). There was marked dilatation of tubules, tubular damage and infiltration of inflammatory cells into the interstitial space. However, kidney section of animals treated with extract (G-III), showed improvement of the above symptoms and reduced crystal deposition as shown in (Figure 3) but significantly improved with Cystone treatment in GIV as shown in (Figure-4) |
| |
Discussion
|
| |
| As traditional medicines are usually taken by the oral route, same route of administration was used for evaluation of antilithiatic effect of the Rotula aquatica against ethylene glycol induced urolithiasis in rats. |
| |
| In the present study, male rats were selected to induce urolithiasis because the urinary system of male rats resembles that of humans (10) and also earlier studies have shown that the amount of stone deposition in female rats was significantly less. (11) Urinary Supersaturation with respect to stoneforming constituents is generally considered to be one of the causative factors in calculogenesis. Evidence in previous studies indicated that in response to 14 day period of ethylene glycol (1% v/v) administration, young male albino rats form renal calculi composed mainly of calcium oxalate.(12, 13) The biochemical mechanisms for this process are related to an increase in the urinary concentration of oxalate. Stone formation in ethylene glycol fed animals is caused by hyperoxaluria, which causes increased renal retention and excretion of oxalate. Similar results have been obtained when rats were treated with ethylene glycol and ammonium oxalate. Therefore, this model was used to evaluate the antilithiatic effect of Rotula aquatica against urolithiasis. (14, 15). |
| |
| In the present study oxalate and calcium excretion progressively increased in calculi- induced animals (G2), since it is accepted that hyperoxaluria, is a far more risk factor in the pathogenesis of renal stones than hypercalciuria (16), the changes in urinary oxalate levels are relatively much more important than those of calcium (17). Increased urinary calcium is a factor favoring the nucleation and precipitation of calcium oxalate (or) apatite (calcium phosphate) from urine and subsequent crystal growth. (18) However Rotula aquatica lower the levels of oxalate as well as calcium excretion. |
| |
| An increase in urinary phosphate is observed in calculi induced rats (G2). Increased urinary phosphate excretion along with oxalate stress seems to provide an environment appropriate for stone formation by forming calcium phosphate crystals, which is epitaxially, induces calcium oxalate deposition. (19) Treatment with Rotula aquatica restored phosphate level, thus reducing the risk of stone formation. |
| |
| The increases in urinary uric acid excretion were observed in urolithiatic rats. Increased excretion of uric acid has been reported in stone formers and hyperoxaluric rats. Uric acid interferes with calcium oxalate solubility and it binds and reduces the inhibitory activity of glycosaminoglycans. The predominance of uric acid crystals in calcium oxalate stones and the observation that uric acid binding proteins are capable of binding to calcium oxalate and modulate its crystallization also suggests its primary role in stone formation. Treatment with Rotula aquatica lowered the excretion of uric acid and reduces the risk of stone formation. |
| |
| Supersaturation, a step in the pathogenesis of nephrolithiasis, occurs when substances that make up the stone are found in the high concentration in urine, when urine volume decreases, and when urinary concentration of chemicals that inhibit stone formation decreases. Inhibitors of crystallization include citrate, magnesium, phosphate; nephrocalcin etc. (20, 21) Low urinary magnesium content is a common feature in stone formers (22). A similar condition was observed in the (G2) rats. Rotula aquatica treatment, elevated the urinary magnesium level, and thus, reduced the propensity to crystallize, thereby creating an ambience unfavorable for precipitation. |
| |
| Increased excretion of proteins has been noted in hyperoxaluric rats and stone formers (23) .A high urinary colloidal concentration favors crystal growth (24). Such a condition was observed with ethylene glycol treated rats, in this study. Administration of the extract reduced the urinary protein excretion in the G3 rats and hence minimizes the conditions favorable for crystal growth. |
| |
| In urolithiasis, the glomerular filtration rate (GFR) decreases due to the obstruction to the outflow of urine by stones in the urinary system. Due to this, the waste products, particularly nitrogenous substances such as creatinine and uric acid get accumulated (25).Also increased lipid peroxidation and decreased levels of antioxidant potential have been reported in the kidneys of rats supplemented with a calculiproducing diet (CPD) (26, 27) .Elevated oxalate has been reported to induce lipid peroxidation and to cause renal tissue damage by reacting with poly unsaturated fatty acids in the cell membrane. (28) In calculi- induced rats (G2), marked renal damage was seen as indicated by the elevated serum levels of creatinine and uric acid. However, treatment with Rotula aquatica causes diuresis and hastens the process of dissolving the preformed stones and prevention of new stone formation in the urinary system. |
| |
| Increase in calcium and oxalate levels in the renal tissue of EG-treated rats were observed. Treatment with Rotula aquatica suppresses this increase in intracellular calcium. This might be due to the increased bioavailability of NO (Nitric Oxide) which in turns activates cGMP (3’, 5’ cyclic guanosine monophosphate) that controls the intracellular rise in calcium levels. Thus, plant extract could effectively control the levels of both the salts by the following mechanism (1) inhibiting the synthesis of oxalate and (2) increasing the bioavailability of NO to sequester calcium through the cGMP pathway. (29). |
| |
| Several studies reported that flavanoids, polyphenols and triterpenes have anti-inflammatory and antioxidant effects. (30, 31, 32)) It can be speculated that antilithiatic activity might be through an antioxidant activity and free radical scavenging principle(s). (33, 34) In GC-MS Spectra Triterpenes- Squaline is identified in Rotula aquatica extract, are having antioxidant properties, (35, 36) so these component might be responsible for antilithiatic activity. |
| |
| Microscopic examination of kidney sections derived ethylene glycol induced urolithiatic rats showed polymorphic irregular crystal deposits inside the tubules which causes dilation of the proximal tubules along with interstitial inflammation that might be attributed to oxalate. Treatment with the Rotula aquatica decreased the number and size of calcium oxalate deposits in different parts of the renal tubules and also prevented damages to the tubules and calyxes. |
| |
Conclusion
|
| |
| In conclusion, the presented data indicate that administration of the Rotula aquatica extracts to rats with ethylene glycol induced lithiasis, reduced and prevented the growth of urinary stones, supporting the folklore claim regarding antilithiatic activity of the plants. The mechanism underlying this effect is still unknown but is apparently related to diuresis and lowering of urinary concentrations of stone forming constituents. The protective effect against oxalate-induced lipid peroxidation may be contributory to the recovery of renal damage. |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










