Key words
|
| |
| Diabetes mellitus; cardiovascular complications; bitter gourd fruit juice (BFJ); glucose; cholesterol; lactate dehydrogenase; left ventricular collagen content; blood pressure |
| |
Introduction
|
| |
| Diabetes mellitus affects an estimated 285 million adults worldwide, of whom approximately 85% to 95% have type 2 diabetes (T2DM) [1]. The heart disease is a major cause of death in diabetic patients [2,3], and coronary artery disease and atherosclerosis are mainly involve in the increased incidence of cardiovascular dysfunction [4,5]. Hypertension and diabetes are interrelated metabolic disorders [6] that strongly predispose an individual to atherosclerotic cardiovascular disease (CVD) [7,8]. The disease causes morbidity and long-term complications and an important risk factor for cardiovascular diseases [9]. Pharmacotherapy of diabetes without any side effects is still a challenge. So that need for complementary and alternative medicine with antidiabetic activity and less side effects. |
| |
| The Bitter melon (Momordica charantia) or Bittergourd fruit (BF), commonly known as karella (L), family: Cucurbitaceae), is grown in tropical countries in South Asia, South America and Africa. The juice of bittergourd fruit has been proved for hypoglycaemic effects in experimental type 1 diabetes and in type 2 human diabetes [10,11]. The juice of bittergourd can increase glucose uptake by tissues in vitro [12]. Many species of the genus in the Cucurbitaceace family, like Momordica Charantia [13, 14,15] and Momordica Cymbalaria [16] have been reported for significant antidiabetic effects. The active fractions from fruits of M. charantia such as saponins [17,18] and peptides [19,20,21] had hypoglycemic effects. In view of various effects of bittergourd fruit juice, this study was designed to assess the effect of bitter gourd fruit juice on glucose tolerance and lipid profile in streptozotocin-induced type –II diabetic rats. |
| |
Material and Methods
|
| |
|
Preparation of Bitter gourd fruit juice
|
| |
| Bitter gourd fruits (BF) were obtained from the local market, washed thoroughly, and fresh juice was prepared on a juicer eliminating seeds. It was centrifuged at 1000 rpm on a tabletop refrigerated centrifuge for 10 min at 4°C. The clear supernatant was considered as 100% Bitter gourd fruit juice (BFJ) which was diluted with autoclaved distilled water to make 25 and 50% juice or any other desired concentrations. The 100% juice was stored at 4°C. [22]. |
| |
|
Experimental animals
|
| |
| Neonatal rats (7–10 g) were used. The experimental protocol was approved by Institutional Animal Ethics Committee and animals were maintained under standard conditions in an animal house approved by Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA). |
| |
|
Induction of diabetes mellitus
|
| |
| Wister rats from an inbred colony were bred under well controlled conditions of temperature (22 ± 2°C), humidity (55 ± 5%) and 12h/12h light-dark cycle. Conventional laboratory diet and tap water were provided ad libitum. Two days old neonates were injected intraperitoneally (i.p.) with 90 mg/kg STZ in citrate buffer solution. Control neonates received equivalent amount of isotonic saline alone. The neonates (n2 STZ rats) were left with their own mothers and weaned at four weeks of age. Twelve weeks after the injection of STZ, animals were checked for fasting glucose levels. |
| |
|
Treatment protocol
|
| |
| Animals showing hyperglycemia (>140 mg/dl serum glucose level), 12 weeks after the injection of STZ were selected for the experiment as diabetics. Control animals injected with 0.1mol/lit citrate buffer were used as normal control. Control rats were randomly divided into 3 groups, similarly, diabetic rats were divided in 3 groups. |
| |
| The experimental animals were divided into 6 groups, six animals in each group. Group-1: Normal control (CON); Group-2: Normal treated with 25% BFJ (10 ml/kg, p.o.) (CBFJ 25); Group-3: Normal treated with 50% BFJ (10 ml/kg, p.o.) (CBFJ 50);Group-4: Diabetic control (DC); Group-5: Diabetic treated with 25% BFJ (10 ml/kg, p.o.) (DBFJ 25); Group-6: Diabetic treated with 50% BFJ (10 ml/kg, p.o.) (DBFJ 50). |
| |
| All the studies were carried for a period of eight weeks. During the study food intake, water intake and body weight of animals were recorded in all the groups. At the end blood samples were collected under fasting conditions, and for glucose tolerance test the animals were orally administered with 1.5 g/kg of glucose and blood samples were collected after 30, 60 & 120 minutes of oral glucose administration. Serum was separated by centrifugation at 5000 rpm for 20 min and stored at –20°C until the analysis was carried out. Serum samples were analyzed for glucose, cholesterol, HDL cholesterol, triglyceride and using diagnostic kits (Span Diagnostics Ltd., India) colorimetrically using UV Visible spectrophotometer (Shimadzu, Japan). VLDL and LDL were calculated as per Friedevald’s equation. |
| |
|
Statistical analysis
|
| |
| Results are presented as Mean + SEM. Statistical differences between the means of the various groups were evaluated using one-way analysis of variance (ANOVA) followed by Tuckey’s test. Data were considered statistically significant at P value < 0.05. |
| |
Results
|
| |
|
General features of experimental rats
|
| |
| Intravenous injection of 90 mg/kg STZ in 2 day old pups rats produced signs of type II diabetes i.e., loss of body weight, polyphagia , and polydipsia after 12 weeks. Treatment with 50% BFJ could reduce the elevated food and water intake of diabetic rats. (Table 1) |
| |
|
Biochemical parameters
|
| |
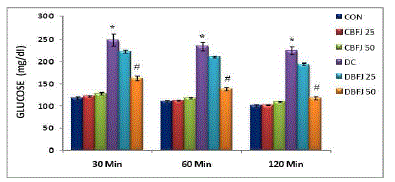
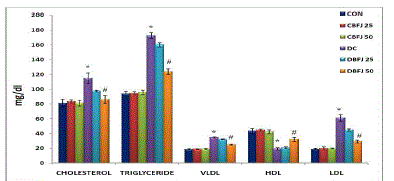
| STZ-diabetic rats were found to exhibit significant (P < 0.05) hyperglycemia compared to control rats in oral glucose tolerance which was improved by 50% BFJ treatment (Table-2) (Figure-1).There were significant (P<0.05) increase in cholesterol, very low density lipoprotein (VLDL), and triglycerides levels, and significant (P<0.05) decrease in high density lipoprotein (HDL)-cholesterol levels in STZ diabetic rats as compared to control rats. Treatment with 50% BFJ significantly (P<0.05) reduced the cholesterol, VLDL and triglyceride levels in diabetic rats and increased the HDL-cholesterol levels. 25%BFJ showed no significant effects on glucose level and lipid profile (Table 3) (Figure-2). |
| |
Discussion
|
| |
| In present study, it was found that there was increase in food & water intake and significant weight loss in diabetic animals as compared to control animals. Treatment with 50%BFJ significantly reduced the elevated food and water intake of diabetic rats. This indicates that 50%BFJ may improve characteristic symptoms of polyphasia & polydypsia of diabetes mellitus. |
| |
| It has been reported that diabetic n2 STZ rats show significant glucose intolerance when orally administered with 1.5 g/kg of glucose and also have high insulin level [23]. In the present study also diabetic animals were found to have impaired glucose tolerance with high glucose level after one hour of glucose load compared to control animals. The results of this study have demonstrated that oral administration of 50% BFJ daily over duration of 8 weeks to STZ-induced type 2 diabetic rats can significantly improve the impaired glucose tolerance compare to 25%BFJ. These results are consistent with those reported earlier [24]. Further it is well known that dietary fibers facilitate slow absorption of glucose in gastrointestinal tract [25,26]. These results support those of Sarkar et al., [27] wherein they have demonstrated the hypoglycernic action of M. charantia in a validated animal model of diabetes. It had been reported that dietary intake of freeze dried bittergourd did not show any beneficial hypoglycemic influence in streptozotocin induced diabetic rats [28]. Perhaps freeze drying process might have led to loss of hypoglycemic activity in these studies. Currently, the cellular mechanisms involved in the hypoglycaemic effects of M. charantia are not yet fully established. However, a number of studies have suggested that M. charantia may either have insulinlike secretagogue effect [29], it can stimulate peripheral glucose utilization or it may inhibit key gluconeogenic enzymes such as glucose-6- phosphatase and fructose biphosphatase [30]. |
| |
| Abnormalities in lipoproteins are very common in both NIDDM and IDDM. Diabetes leads to alterations in the plasma lipid and lipoprotein profile and increases risk of coronary heart disease [31]. In patients with type 2 diabetes hyper triglyceridemia and low HDL-cholesterol levels are common [32]. Reducing the serum lipid levels through dietary or drug therapy decrease in the risk of cardiovascular disease and related complications [33].In addition to the hypoglycemic activity of BFJ, it also possesses lipid lowering properties in diabetic animals. In the present investigation, serum cholesterol and triglyceride levels of diabetic rats were found to be significantly decreased by the treatment with 50%BFJ. We have also observed that in STZ induced diabetic rats; the level of HDL-cholesterol was significantly lower. 50%BFJ normalized these effects, possibly by controlling the hydrolysis of certain lipoproteins and their selective uptake and metabolism by different tissues. The strong antihyperlipidemic effect of BFJ could also be through its control of hyperglycemia, as this is a major determinant of total and very low density lipoprotein (LDL) and triglyceride concentration [34].Framingham study suggested that high plasma triglycerides is one of the risk factors for coronary heart disease in women [35] and in case of men, it is the risk factor when hyperlipidemia is associated with low levels of HDL-cholesterol concentration [36].Thus lowering of cholesterol and triglyceride levels with increase in the HDL-cholesterol by 50%BFJ may produce beneficial effects on STZinduced cardiovascular complications. The results of BFJ highlight the topic for research and discussion for dose dependent effects of BFJ. |
| |
Conclusion
|
| |
| Our data suggest that BFJ prevents the STZ induced metabolic abnormalities as evident from the reduction in cholesterol, triglyceride, In conclusion, the results of this study have clearly demonstrated that bittergourd fruit juice can have marked beneficial effects in the treatment of diabetes mellitus, bittergourd fruit juice administration may be useful as an adjunct therapy with oral hypoglycaemic agents in the management of diabetes mellitus. |
| |
Acknowledgment
|
| |
| We like to acknowledge “Sat Kaival College of Pharmacy” for support. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |








