Keywords
|
| Innominate artery stenosis; Middle cerebral artery occlusion; Endovascular therapy; Embolectomy; Neurosonography; Endarterectomy |
Introduction
|
| Embolic stroke due to stenosis of the innominate artery (IA), or brachiocephalic trunk is a rare entity [1-2]. The most common cause of IA disease is atherosclerosis followed by Takayasu’s arteritis, dissection, thoracic outlet syndrome, radiotherapy-induced supra-aortic stem disease, mural thrombus of ascending aorta, nephrotic syndrome and fibromuscular dysplasia. High-grade IA stenosis (IAS) or occlusion can cause inherent dynamic changes in cerebral blood flow with flow reverseal in both, the vertebral and carotid arteries and is sometime called double subclaviancarotid steal syndrome, or the innominate steal syndrome [3-13]. While symptoms are often transient due to the hemodynamic nature diagnosis can be made using noninvasive methods such as Doppler ultrasound, angiographic examinations and bilateral blood pressure measurements. In rare cases of plaque rupture with subsequent thromboembolism may cause right middle cerebral or posterior circulation infarctions. |
| We present a case of successful endovascular right middle cerebral (MCA) embolectomy in a patient with undetected previously asymptomatic IAS one month after coronary artery bypass surgery. IAS was diagnosed prior the acute revascularisation procedure, did not hinder embolectomy and was subsequently treated by means of open endarterectomy. |
Case report
|
| A 67 year old right-handed male admitted to the stroke unit due to sudden onset of left sided hemiparesis within two hours from the onset of symptoms. Vital signs showed only mild hypertension while the neurological examination revealed left side strength 0/5 per Medical Research Council scale, hemineglect with head and eye deviation to the right side. The National Institute of Health Stroke Scale (NIHSS) score was 12/42. Current medication consisted of antihypertensive only. |
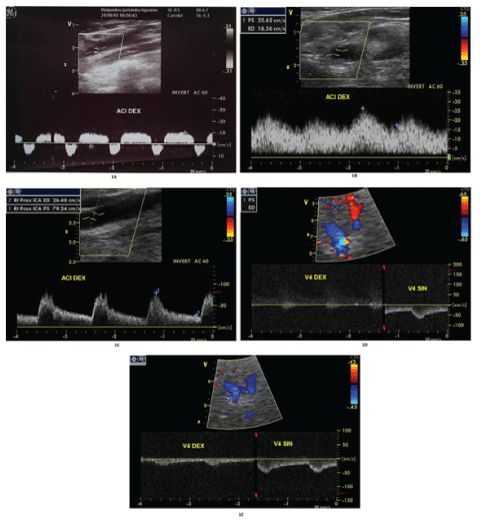
| Cerebral computed tomography (cCT) excluded brain haemorrhage and no early signs of cerebral ischemia. Blood counts and blood chemistry were within normal range. Systemic thrombolysis was excluded due to coronary artery bypass surgery (CABG) one month ago. Emergency extracranial colour-coded duplex sonography (ECCS) excluded significant internal carotid artery (ICA) disease, however, a highly pathological alternating blood flow pattern right ICA (Figure 1A) complete reversal of flow in the right vertebral artery (VA) suggestive of high-grade stenosis or occlusion of brachiocephalic trunc. |
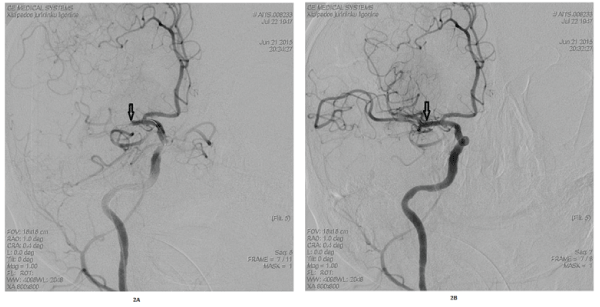
| Transcranial colour-coded duplex sonography (TCCS) showed a pre-occlusive blood flow of the right MCA main stem and suspected complete occlusion of distal M1. Emergency selective digital subtraction angiography (DSA) of the carotid arteries confirmed the occlusion of the MCA (Figure 2A). Given the moderate-to-severe clinical symptoms despite MCA occlusion and lack of early signs of cerebral infarction,endovascular embolectomy using the Solitaire® stent retriever was performed. |
| The procedure was performed under mild sedation. MCA blood flow was completely restored after 4 hours from the onset of symptoms (mTICI, modified Treatment in Cerebral Ischaemia Scale [14], was 3) (Figure 2B). The following day, cCT revealed acute ischemia in the right basal ganglia but no hemispheric infarction. |
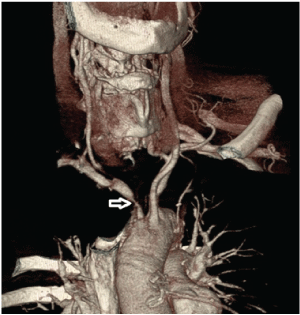
| The neurological condition of the patient improved significantly with only mild left hemiparesis after one week (NIHSS 4/42). The repeated ultrasound examination revealed post-stenotic (dampened) blood flow in the right carotid artery (Figure 1B), while the right vertebral artery still had retrograde blood flow (Figure 1D). TCCS showed changes in the right MCA from pre-occlusive to now post-stenotic. CT angiography (CTA) of the aortic arch confirmed critical atherosclerotic stenosis of IA (Figure 3). IA endarterectomy was performed for secondary stroke prevention and the intraoperative picture revealed subocclusion by soft atherosclerotic masses. Neurovascular follow-up revealed normal blood flow through the right carotid and vertebral arteries (Figures 1C and 1E). |
| Medical treatment post stroke and CABG consisted of platelet inhibition, statin therapy and blood-pressure control, and the patient subsequently referred to neurorehabilitation. He was discharged 1 month later without significant disability (modified Rankin Scale 1). |
Discussion
|
| We here demonstrate a case of embolic MCA occlusion from significant IAS that was successfully treated using endovascular embolectomy. While IA disease itself is a rare condition it does not preclude from endovascular approaches and, given the pre-existing hemodynamic compromise, may even lead to ischemic pre-conditioning with good collateral flow development and thus better outcome upon emergency treatment. |
| Selective angiography is the gold standard for an accurate assessment of IAS, but non-invasive studies such as extra- and intracranial Duplex sonography and CT/MR angiography dominate the diagnostic strategy. An early extensive arteriographic study estimated that only 4% of patients with cerebrovascular insufficiency had innominate artery disease that most often present with transient/ reversible neurological symptoms in the posterior circulation upon the hemodynamic compromise [1]. It may present with the symptoms of ischemic disease of the right arm and brain, usually posterior circulation. The most common source IAS derived emboli is a local plaque rupture and, less frequently, dissection, thrombosis due to mechanical effects, radiation, hypercoagulation and mural thrombus of ascending aorta [4-11]. MCA occlusion from IAS, as in our case, is the most likely pathophysiology given the intra-operative finding of fresh thrombotic material in the innominate artery. However, we cannot completely rule out intermittent atrial fibrillation, a condition not rare after CABG although diagnostic work-up was negative. Remarkably, patients should be scanned for concomitant neurovascular disease prior CABG which has not been done in this particular case. |
| An interesting aspect of the embolectomy procedure itself is the unproblematic passage of the catheters through IAS during the emergency embolectomy. Therefor no emergency stent placement with subsequent aggressive platelet inhibition was necessary. After embolectomy and recanalisation of the MCA, ischemia was seen only in the right area of basal ganglia. This remarkable outcome may be due to ischemic preconditioning from the preceding hemodynamic compromise. The concept of ischemic preconditioning in stroke patients normally utilizes “remote” ischemia, normally of the limb [15]. However, experimental strategy target i.e., hypoxia-inducible factor, sphingosine kinase 2 and chemokine ligand 2 to induce a gene expression responsible for the stroke-tolerant phenotype [16]. Also, IAS might have promoted the development of pial collateral network by upregulation of vascular endothelial growth factor, all was which might have contributed to the excellent outcome [17]. Recently endovascular thrombectomy trials and CTA/CT perfusion studies showed that patients with ischemic preconditioning and good collaterals have much better outcomes associated with rapid recanalization and reduction of infarct size after interventional treatment of acute large intracerebral artery occlusion [18-20]. |
| Our clinical case demonstrates the value of neurovascular ultrasound in the acute phase of stroke including the selection of appropriate candidates for neurovascular embolectomy. However, extra- and intracranial Duplex sonography have not been used in the large embolectomy trials. CT- and MRangiography are the preferred diagnostic procedure in large intracranial artery occlusion, but high quality ultrasound may still have a profound value in case other CT-A is not possible due to uncooperative patient, imminent renal failure or for technical reasons. |
| Due to its rarity, there are no evidence based guidelines for IAS treatment. Of late, angioplasty with or without stenting has been favoured, yet vascular surgery is recommended in patients subsequently undergoing CABG at the same time [21]. Given the recent stroke, yet with minor basal ganglia infarction, our case multidisciplinary team choose the surgical treatment over stenting in order to avoid further dislocation of thrombus material into the cerebral circulation and also to evade from double platelet therapy in the early post-stroke phase. |
| Research studies describe only isolated cases of specific treatment of acute cerebral infarction in the carotid circulation caused by symptomatic atherosclerotic IA disease [22-24]. Two cases of occlusion of the MCA stem treated with intravenous thrombolysis and one case of occlusion of distal ICA treated with mechanical thrombectomy have been published: in the case of Youn et al. [22], no recanalization upon IV thrombolysis occured, while in the case of Sanchez-Ayaso et al. [23] only partial MCA recanalization was obtained. Gordhan et al. described the case of successfully applied carotid “T” occlusion treatment by mechanical thrombectomy/ thromboaspiration with complete recanalization and with a good neurological outcome [24]. The latter case is similar to our case. |
| In summary, MCA embolectomy from IAS embolization is a rare entity but may result in a good clinical outcome perhaps due to ischemic preconditioning. Assuming the rareness of the disease, an international registry of similar cases would be helpful for better understanding the IA disease and guiding acute interventions. |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |
|
|








