Keywords
Emerging Infectious Diseases, travel medicine, tropical diseases, SARS, Avian Flu, Influenza, Chikungunya
Introduction
The field of infectious disease is vast, complex and rapidly expanding. New advances in diagnostic testing (eg. polymerase chain reaction-PCR technology), the development of new antimicrobial therapies [1] (eg. antiretroviral medications), and even the emergence of novel infectious diseases (eg. severe acute respiratory syndrome (SARS) requires diligent study by medical practitioners to remain current. This demands great effort from the practitioner of emergency medicine who must maintain up to date medical knowledge in many fields simultaneously. The number of people travelling internationally is increasing every year [2].
According to statistics of the World Tourism Organization, international tourist arrivals for 2009 exceeded 840 million. In 2009, the majority (410 million) of international tourist arrivals were for the purposes of leisure, recreation and holiday (51%) [2]. Therefore, the importance of travel medicine becomes obvious and its contribution to public health essential. International travel can pose various risks to health, depending on the characteristics of both the traveller and the travel. Travellers may encounter sudden and significant changes in altitude, humidity, microbes and temperature, which can result in ill-health.
In addition, serious health risks may arise in areas where accommodation is of poor quality, hygiene and sanitation are inadequate, medical services are not well developed and clean water is unavailable. This review article’s purpose is to highlight the details and impact of four significant diseases that pose a threat to public health. Specifically we will talk about issues such as the cause, transmission, geographical distribution, nature and precautions of SARS, Influenza, Chikungunya and Avian Flu.
Background
Important health organizations with a national or worldview of disease (e.g., the Centers for Disease Control and Prevention and the World Health Organization) have restructured themselves to place greater emphasis on infectious diseases and rapid responses to epidemics, wherever they emerge. Developed nations, recognizing the threat to their economic welfare and social stability, have poured increasing resources into prevention, detection, surveillance, and treatment of emerging infectious diseases and pledged to fight infectious diseases around the world in various cooperative efforts. Funds to support research aimed at understanding and controlling emerging infectious diseases have increased significantly over the past decade as governments have recognized the importance of this threat [2].
In addition, we focus on specific emerging infectious diseases of increasing interest to both the scientific community and the general public, as SARS, Influenza, Chikungunya and Avian Flu. For these diseases we examine the epidemiology (Cause, Transmission, and geographical distribution), clinical picture (nature of disease), treatment and prevention (Risk for travellers, Prophylaxis, Precautions).
Sars
Severe acute respiratory syndrome (SARS) is an often fatal infectious respiratory disease with prominent systemic symptoms. It is caused by a novel coronavirus, SARS coronavirus(SARS-CoV), which was responsible for a global outbreak from November 2002 to July 2003. SARS-CoV is thought to be an animal virus from an as–yet uncertain–animal reservoir, perhaps bats, that spread to other animals (civets) and first infected humans in the Guangdong province of southern China in 2002 [3-6]. SARS-CoV is a zoonosis that initially affected wild animals, possibly bats, and subsequently spread to exotic animals. The virus can be identified by reverse transcriptase polymerase chain reaction (RT-PCR) in blood, plasma, respiratory secretions, and stool. Specific antibody is detected in acute and convalescent sera from patients by indirect fluorescent antibody (IFA) testing and enzyme-linked immunosorbent assay (ELISA) targeting the surface spike (S) protein.
An epidemic of SARS affected 26 countries and resulted in over 8000 cases in 2003. Since then, a small number of cases have occurred as a result of laboratory accidents or, possibly, through animal-to-human transmission (Guangdong, China) [3-6]. Transmission of SARS-CoV is primarily from person-to-person. It occurs mainly during the second week of illness, which corresponds to the peak of virus excretion in respiratory secretions and stool, and when cases with severe disease start to deteriorate clinically.
Symptoms are flu-like and include fever, malaise, muscle aches and pains (myalgia), headache, diarrhoea, and shivering (rigors). No individual symptom or cluster of symptoms has proved to be specific for a diagnosis of SARS [3,4]. Although fever is the most frequently reported symptom, it is sometimes absent on initial measurement, especially in elderly and immunosuppressed patients. Cough (initially dry), shortness of breath, and diarrhoea present in the first and/or second week of illness. Severe cases often develop rapidly, progressing to respiratory distress and requiring intensive care (see fig 1).
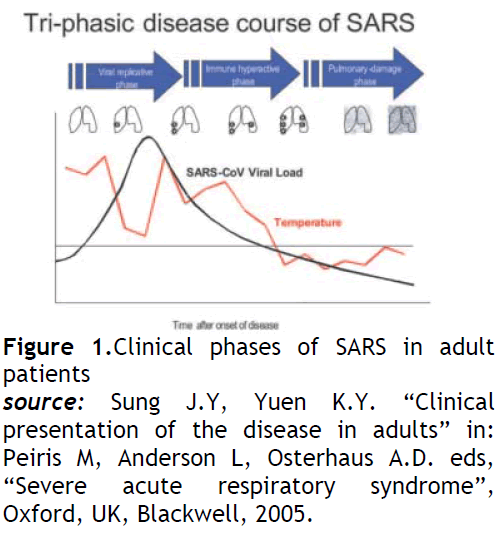
Figure 1: Clinical phases of SARS in adult patients
source:Sung J.Y, Yuen K.Y. “Clinical presentation of the disease in adults” in: Peiris M, Anderson L, Osterhaus A.D. eds, “Severe acute respiratory syndrome”, Oxford, UK, Blackwell, 2005.
The distribution is based on the 2002–2003 epidemic [5]. The disease appeared in November 2002 in the Guangdong province of southern China. This area is considered as a potential zone of re-emergence of SARS-CoV [5]. Other countries/areas in which chains of human-to-human transmission occurred after early importation of cases were Hong Kong Special Administrative Region of China and Taiwan (Province of China), Toronto in Canada, Singapore, and Hanoi in Vietnam. In other countries, imported cases did not lead to local outbreaks.
Currently, no areas of the world are reporting transmission of SARS. Since the end of the global epidemic in July 2003, SARS has reappeared four times – three times from laboratory accidents (Singapore; Taiwan, Province of China), and once in southern China where the source of infection remains undetermined although there is circumstantial evidence of animal-to-human transmission [7-9]. Should SARS re-emerge in epidemic form, WHO will provide guidance on the risk of travel to affected areas. Travellers should stay informed about current travel recommendations. However, even during the height of the 2003 epidemic, the overall risk of SARS-CoV transmission to travellers was low.
There in no prophylaxis. Experimental vaccines are under development.
The precautions impose to follow any travel recommendations and health advice issued by WHO [6-9]. Four specific measures are important in the infection control practice for SARS: hand washing and the wearing of masks, gowns, and gloves. The quantity of exposure is related to the duration of hospital stay of SARS patients. A longer exposure results in higher chance for procedural lapses to occur, which can result in nosocomial spread [9,10].
1. Patient-related risk (exposure to a confirmed or suspected SARS case, superspreading events, triage areas, patient with fever of unknown origin, etc.)
2. Procedure-related risk (ICU, procedure room such as bronchoscopy room or x-ray department, area serving SARS patients, dirty utility room, etc.)
3. Direct patient contact or activities with risk of exposure to blood, body fluids, secretions, excreta, and contaminated items.
4. In addition, procedures with high risk of generating aerosols (e.g., resuscitation, high-flow oxygen) and involving prolonged very close contact with affected patients require:
• N95 respirator (surgical mask may suffice for non-aerosol generating procedures)
• a linen or disposable gown
• full-face shield or eye shield
• Latex gloves (only for procedures with exposure to blood and body fluid, secretion, excreta, and contaminated items)
• goggles (only for aerosol-generating procedures)
• disposable cap (optional)
Influenza
An influenza virus of types A, B and C. Type A occurs in two principal subtypes (H1N1 and H3N2). Type A viruses cause most of the widespread influenza epidemics, type B viruses generally cause regional or sporadic outbreaks, and type C cause mild disease in the form of common colds and bronchitis in children. Influenza viruses evolve rapidly, changing their antigenic characteristics, so that vaccines need to be modified each year to be effective against currently circulating influenza strains [11,12]. Other subtypes of influenza A viruses occur in animals and all 16 HA and 9 NA subtypes are found in birds (mainly in water fowl) inter-species transmission (1918 pandemic) and viral reassortment (1957, 1968 pandemics) may give rise to new subtypes able to infect humans. Influenza is usually a self-limited, acute viral respiratory illness that has the potential to cause significant morbidity and mortality of global dimensions. It typically occurs in regional epidemics lasting 8 to10 weeks during the winter months. During an average epidemic in the United States, approximately 30,000 deaths occur in excess of what would be normally observed. Put in perspective, influenza kills more Americans every year than any other infectious disease including AIDS. Every decade or so, changes in the virulence and antigenic makeup of influenza viruses permit these viruses to span the globe in a nonseasonal pandemic characterized by a high attack rate and a mortality rate in excess of three times that seen during a typical year. The most dramatic example of a severe pandemic occurred between 1918 and 1919, when the "Spanish flu" killed approximately 700,000 Americans during a 12-week period. This is even more dramatic when one considers that in 1918 the U.S. population was only 1/3 of what it is today and that most of the deaths occurred among young adults between the ages of 20 and 45 years. It is estimated that as many as 50 million persons worldwide died during this same period [12].
Respiratory transmission occurs mainly by droplets disseminated by unprotected coughs and sneezes. Short-distance airborne transmission of influenza viruses may occur, particularly in crowded enclosed spaces. Hand contamination and direct inoculation of virus is another potential route of spread.
The nature of the disease is that it is an acute respiratory infection of varying severity, ranging from asymptomatic infection to fatal disease [13]. Classic influenza symptoms include fever with rapid onset, sore throat, cough and chills, often accompanied by headache, coryza, myalgia and prostration. Influenza may be complicated by viral or more often bacterial pneumonia. Illness tends to be most severe in the elderly and in infants and young children [14], and in immunocompromised hosts. Death resulting from seasonal influenza occurs mainly in the elderly and in individuals with pre-existing chronic diseases.
Its geographical distribution is Worldwide [15-17]. In temperate regions, influenza is a seasonal disease occurring typically in winter months: it affects the northern hemisphere from November to April and the southern hemisphere from April to September. In tropical areas there is no clear seasonal pattern, and influenza may occur at any time of the year. Activity may occur year-round in the tropics.
Travellers, like local residents, are at risk in any country during the influenza season. In addition, groups of travellers that include persons from areas affected by seasonal influenza (e.g. cruise ships) may experience out-offseason outbreaks. Travellers visiting countries in the opposite hemisphere during the influenza season are at special risk, particular if they do not have some degree of immunity through regular vaccination. The elderly, people with pre-existing chronic diseases and young children are most susceptible to complications.
The prophylaxis is vaccination (see fig 2), and it should start before the of the influenza season [18,19]. However, vaccine for visitors to the opposite hemisphere may not be obtainable before arrival at the travel destination. For travellers in the highest risk groups for severe influenza who have not been or cannot be vaccinated, the prophylactic use of antiviral drugs such as zanamivir or oseltamivir is indicated in countries where they are available. Amantadine and rimantadine may also be considered when the circulating strains are known to be susceptible. However, the latter drugs are not active against influenza B, and high frequencies of resistance in H3N2 and less often H1N1 viruses make then unreliable for prevention currently.

Figure 2: Flu Vaccine instructions.
Source: CDC (2006)
Precautions that should be taken whenever possible, are to avoid crowded enclosed spaces and close contact with people suffering from acute respiratory infections [20]. Hand-washing after direct contact with ill persons or their environment may reduce the risk of illness [21]. Ill persons should be encouraged to practise cough etiquette (maintain distance, cover coughs and sneezes with disposable tissues or clothing, wash hands).
Chikungunya
Chikungunya virus is an Alphavirus (Togavirus family). Chikungunya Virus (CHIKV) was first isolated during a 1952 epidemic in Tanzania. Chikungunya comes from Swahili, meaning “that which bends up,” and refers to the characteristic posture assumed by patients typically suffering severe joint pains. Carey [22] presented convincing historical evidence that CHIKV has occurred sporadically in India and Southeast Asia for at least 200 years. The known geographic distribution of the virus includes most of sub-Saharan Africa, India, Southeast Asia, Indonesia, and the Philippines [23,24].
Chikungunya is transmitted by mosquitoes, including several Aedes species which bite during daylight hours. Two important vectors are Aedes aegyptiand Aedes albopictus, both of which also transmit dengue virus. There is no direct person-to-person transmission. The virus has been isolated from monkeys in Africa.
Chikungunya is an acute febrile illness with sudden onset of fever and joint pains, particularly affecting the hands, wrists, ankles and feet [25]. There may be severe chills, leukopenia and often a rash. Generalized myalgia is also common. Most patients recover after a few days but in some cases the joint pains may persist for weeks, months or even longer. Chikungunya may also be asymptomatic.
Chikungunya occurs in sub-Saharan Africa, South-East Asia and tropical areas of the Indian subcontinent, as well as islands in the south-western Indian Ocean [26,27]. The period 2005–2007 witnessed an unprecedented spread of outbreaks that affected at least 12 countries and territories (see fig 3), including a localized outbreak in north-eastern Italy.
Source: WHO (2008)
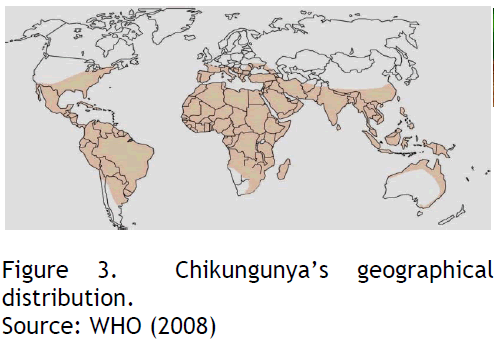
Figure 3: Chikungunya’s geographical distribution.
Source: WHO (2008)
There is a risk for travellers in areas where chikungunya is endemic and in areas affected by epidemics. Also, there is no Prophylaxis and the travellers should take precautions to avoid mosquito bites both during the day and night.
Avian Influenza
Since 2003, the global panzootic of highly pathogenic avian influenza A (H5N1) among domestic poultry and wild birds has resulted in rare, sporadic, human H5N1 cases of severe respiratory disease with high mortality in Asia, Europe, the Middle East, and Africa [28]. Family clusters of H5N1 cases have been documented, and though most transmission of H5N1 viruses to humans is believed to be directly from sick or dead birds, limited human-to-human transmission of H5N1 viruses has been reported. As H5N1 viruses continue to evolve, the concern for a global influenza pandemic rises. Avian influenza A (H5N1) virus, and sometimes other non-human influenza subtypes (H7, H9).
Human infections with avian influenza A(H5N1) virus occur through bird-to-human, possibly environment-to-human, and very rarely limited, non-sustained human-to-human transmission [29]. Direct contact with infected poultry, or surfaces and objects contaminated by their droppings, is the main route of spread to humans. Exposure risk is considered highest during slaughter, de-feathering, butchering, and preparation of poultry for cooking. There is no evidence that properly cooked poultry or poultry products can be a source of infection.
Patients usually present initially with symptoms of fever and influenza-like illness (malaise, myalgia, cough, sore throat). Diarrhoea and other gastrointestinal symptoms may occur. The disease progresses within days and almost all patients develop clinically apparent pneumonia with radiographic infiltrates of varying patterns. Sputum production is variable and sometimes bloody. Multiorgan failure, sepsis-like syndromes, and uncommonly encephalopathy, occur. The fatality rate among hospitalized patients with confirmed H5N1 infection has been high (about 60%), most commonly as a result of respiratory failure caused by progressive pneumonia and acute respiratory distress syndrome [30,31].
Extensive outbreaks in poultry have occurred in parts of Asia, the Middle East, Europe and Africa since 2003, but only sporadic human infections have occurred to date. Continued exposure of humans to avian H5N1 viruses increases the likelihood that the virus will acquire the necessary characteristics for efficient and sustained human-to-human transmission through either gradual genetic mutation or reassortment with a human influenza A virus [30-32].
Between November 2003 and October 2007, > 300 human cases of laboratory-confirmed H5N1 infection were reported to WHO from 12 countries in South-East and central Asia, Europe, Africa and the Middle East [32].
H5N1 avian influenza is primarily a disease in birds. The virus does not easily cross the species barrier to infect humans. To date, no traveller has been infected. The risk of infection is increased by prolonged, close and heavy exposure to the virus.
No human H5 vaccine is commercially available at presen [33]. Neuraminidase inhibitors (oseltamivir, zanamivir) are inhibitory for the virus and demonstrate proven efficacy in vitro and in animal studies for prophylaxis and treatment of H5N1 infection. They are therefore recommended for post-exposure prophylaxis in certain exposed persons: At present WHO does not recommend pre-exposure prophylaxis for travellers but advice may change depending on new findings.
Travellers should avoid contact with high-risk environments in affected countries such as live animal markets and poultry farms, any free-ranging or caged poultry, or surfaces that might be contaminated by poultry droppings. Travellers in affected countries should avoid contact with dead migratory birds or wild birds showing signs of disease. Travellers should avoid consumption of undercooked eggs, poultry or poultry products. Hand hygiene with frequent washing or use of alcohol rubs is recommended. If exposure to persons with suspected H5N1 illness or severe, unexplained respiratory illness occurs, travellers should urgently consult health professionals. Travellers should contact their local health providers or national health authorities for supplementary information [34].
Infection control for suspected and confirmed H5N1 patients includes three key steps: isolation of the patient, use of appropriate personal protective equipment (PPE) for health care workers and visiting family members, and adherence to infection control precautions. Suspected and confirmed H5N1 patients should be isolated in a single room with a controlled entryway. Health care workers and family members should be equipped with disposable gowns, gloves, goggles, and surgical masks or, if available, fit-tested respirators (e.g., N95). Use of respirators is especially important when performing tracheal suctioning or aerosol-generating procedures such as intubation and administration of aerosolized bronchodilator medications. Standard, contact, droplet, and air-borne precautions should be observed as much as possible. Negative-pressure rooms can be used if available but are not required. Proper donning and removal of contaminated PPE, safe disposal of contaminated PPE, and hand washing are essential.
Discussion
Emergency physicians play an important role in the recognition and control of emerging infectious diseases35. As in other aspects of the practice, pattern recognition is the cornerstone of diagnosis. Emergency physicians must be familiar with the clinical presentation of currently emerging infectious diseases in their region as well as nationally and internationally. To be able to promptly activate the public health system one must recognize new, different, and unusual patterns of disease presentation in the emergency department. Key components of the history that must be obtained include travel and potential occupational, dietary, or recreational exposures. The emergency physician must direct appropriate patient isolation and take precautions to protect oneself, the staff, and all other persons in the treatment area.
Emerging infections are, by definition, rapidly changing. The usual sources of medical information, peer-reviewed articles and medical textbooks, generally will not provide adequate information. The practicing emergency physician must be aware of local and national news and should frequently obtain up-to-date information through the Web site of the Center for Disease Control and Prevention or World Health Organization.
The combination of changing ecology and travel and transport of organisms is best seen clearly in antibacterial resistance. Sometimes, the distance travelled is only from one bed to another in the intensive care unit, but in other cases the movement has been worldwide [35].
All people planning travel should know about the potential hazards of the countries they are travelling to and learn how to minimize their risk of acquiring these diseases. Forward planning, appropriate preventive measures and careful precautions can substantially reduce the risks of adverse health consequences. Although the medical profession and the travel industry can provide a great deal of help and advice, it is the traveller’s responsibility to ask for information, to understand the risks involved, and to take the necessary precautions for the journey.
Health professionals and the general public now understand that, with respect to infectious diseases, we live in a global community in which the health of developed and developing nations is intertwined. In this global community, infectious diseases can spread rapidly around the world, making global surveillance and control of emerging infections vital to world health [36-39].
These organisms may migrate from one part of the world to another on wind currents, among migratory fowl, or among travellers. Exotic infectious diseases on distant continents, once seen as posing little threat to the developed world, are now viewed with growing concern among scientists worldwide. Ecological changes and changes wrought by peoples of the world have contributed to the emergence of infectious diseases. These “advances of mankind” include movement of humans and domestic animals into new habitats, deforestation, irrigation, urbanization, and increased air travel. Moreover, public policy and politics—for example, decisions influencing poverty, economic development, population movements, refugees and migrants, and cooperation among governments that can exert enormous influence on the detection, surveillance, treatment, and prevention of emerging infectious diseases—also shape patterns of emerging and reemerging infectious diseases [40]. Individual and group behaviours heavily influence transmission of infectious diseases. In addition, the global decline of public health systems serves to render the world more susceptible to infectious diseases of all kinds [38-41].
Conclusions
Important health organizations with a national or worldview of disease (e.g., the Centers for Disease Control and Prevention and the World Health Organization) have restructured themselves to place greater emphasis on infectious diseases and rapid responses to epidemics, wherever they emerge. Developed nations, recognizing the threat to their economic welfare and social stability, have poured increasing resources into prevention, detection, surveillance, and treatment of emerging infectious diseases and pledged to fight infectious diseases around the world in various cooperative efforts. Funds to support research aimed at understanding and controlling emerging infectious diseases have increased significantly over the past decade as governments have recognized the importance of this threat.
This article hopes to provide readers the knowledge to better understand the emergence and re-emergence of infectious diseases. The future is upon us, and how we prevent and respond to outbreaks of emerging infectious diseases will influence our social-economic, and political status as well as our security for the coming decades. The global village is a reality indeed. When an event affects the population in one part of our world, it inevitably will have an impact on all others.
‘Virtually any destination can be reached from any other in only 36 hours of travel. This 36-hour window is well within the incubation period of most infectious diseases, thus providing ample opportunity for disease transmission to travellers and by them.
3395
References
- KyriakiKanellakopoulou, PavlosSarafis, Irene Galani, Helen Giamarellou, Evangelos J. Giamarellos-Bourboulis In vitro synergism of β-lactams with ciprofloxacin and moxifloxacin against genetically distinct multidrug-resistant isolates of Pseudomonas aeruginosa International Journal of Antimicrobial Agents – 2008,32(1): 33-39.
- Lashley F, Durham J.D. “Emerging Infectious Diseases. Trends and Issues” Second Edition, Springer Publishing Company, New York, 2009: 1-6.
- Antonio G.E, Wong K.T, Hui D. S. C, Lee N, Yuen E, Wu A..“Imaging of severeacute respiratory syndrome in Hong Kong”, AJR 2003, 181:11–7
- Booth C.M, Matukas L.M, Tomlinson G.A, Rachlis A. R, Rose D.B, Dwosh H.A. “Clinical features and short-term outcome of 144 patients with SARS in the greater Toronto area”, JAMA 2003, 289: 2801–9.
- Centers for Disease Control and Prevention (CDC). “Severe acute respiratory syndrome: revised CSTE SARS Surveillance case definition”, 3 May, 2005. Available at: https://www.cdc.gov/ncidod/SARS/guidance/b/app1.htm. Accessed on October 31, 2010.
- World Health Organization (WHO). “Case definitions for surveillance of severe acute respiratory syndrome (SARS)” 1 May 2003. Available at: https://www.who.int/csr/sars/casedefinition/en. Accessed on October 31, 2010.
- World Health Organization (WHO). “Consensus document on the epidemiology of severe acute respiratory syndrome (SARS)”. Available at: https://www.who.int/csr/sars/en/WHOconsensus.pdf. Accessed on October 31, 2010.
- World Health Organization (WHO). “Summary of probable SARS cases with onset of illness” from 1 November 2002 to 31 July 2003. www.who.int/csr/sars/country/table2004˙04˙21/en/index.html. October 31, 2010.
- Peiris M, Anderson L.J, Osterhaus A.D.M.E, Stohr K. “Severe acute respiratory syndrome”, Oxford, UK, Blackwell, 2005.
- Sung J.J.Y. “Severe acute respiratory syndrome: from bench top to bedside”. Singapore, World Scientific, 2004.
- World Health Organization (WHO). “International travel and health 2008”, chapter 5, “Infectious Diseases of potential risk for travellers”, pp 53-80. Available at: https://www.who.int/ith/chapters/en/index.html. Accessed on August 31, 2010.
- Barry J.M. “The Great Influenza”, New York, Penquin Group, 2004:546.
- De Jong J.C, Rimmelzwaan G.F, Fouchier R.A, Osterhaus A.D.M.E. “Influenza virus:A master of metamorphosis”, J Infect 2000,40:218-228.
- Grose C. “The puzzling picture of acute necrotizing encephalopathy after influenza A and B virus infection in young children”, Pediatr Infect Dis J 2004,23:253-254.
- Hilleman M.R. “Realities and enigmas of human viral influenza: Pathogenesis, epidemiology and control”, Vaccine 2002,20:3068-308.
- Nicholson K.G, Wood J.M, Zambon M. “Influenza”, Lancet 2003,362:1733-174.
- Rao B.L. “Epidemiology and control of influenza”, Natl Med J India 2003,16:143-149.
- Kaufmann A, Salentin R, Meyer R.G, Bussfeld D, Pauligk C, Fesq H. “Defense against influenza A virus infection: Essential role of the chemokine system”, Immunobiology 2001,204:603-61.
- Fleming D.M. “Influenza diagnosis and treatment: A view from clinical practice”, Phil Trans R Soc B 2001,356:1933-1943.
- Studahl M. “Influenza virus and CNS manifestations”, J ClinVirol 2003,28:225-232.
- Centers for Disease Control. “Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP)”, Morb Mortal Wkly Rep 2004,53:1-40.
- Carey D.E. “Chikungunya and dengue: a case of mistaken identity?” J Hist Med Allied Sci 1971, 26:243.
- Tesh R.B. “Arthritides caused by mosquito-borne viruses”, Ann Rev Med 1982,33:31.
- Tesh R.B, Gajdusek D.C, Garruto R.M. “The distribution and prevalence of Group Aarbovirus neutralizing antibodies among human populations in Southeast Asia and the Pacific Islands”. Am J Trop Med Hyg 1975,25:664.
- Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. “Vectors of Chikungunya virus in Senegal: Current data and transmission cycles”, Am J Trop Med Hyg 1999, 60:281.
- Powers A.M, Brault A.C, Tesh R.B, Weaver SC. “Re-emergence ofChikungunya and o’nyong-nyong viruses: Evidence for distinct geographical lineages and distant evolutionary relationships”, J Gen Virol 2000,81:471.
- Tsai T.F, Weaver S.C, Monath T.P. “Alphaviruses”, in Richman D.D, Whitley R.J, Hayden F.G. (eds): “Clinical Virology”, Washington, DC: ASM Press, 2002, pp :1177.
- Tran T.H, Nguyen T.L, Nguyen T.D, Luong T.S, Pham P.M, Nguyen VC. “World Health Organization International Avian Influenza Investigative Team. Avian influenza A (H5N1) in 10 patients in Vietnam”, N Engl J Med 2004,350:1179–88.
- Ungchusak K, Auewarakul P, Dowell S.F, Kitphati R, Auwanit W, Puthavathana P. “Probable person-to-person transmission of avian influenza A(H5N1)”, N Engl J Med 2005,352:333–40.
- World Health Organization (WHO). Update: “WHO-confirmed human cases of avian influenza A(H5N1) infection”, 25 November 2003–24 November 2006. WklyEpidemiol Re 2007,82:41–8.
- World Health Organization (WHO).Writing Committee of the Second World Health Organization (WHO) Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus, “Update on avian influenza A (H5N1) virus infection in humans”, N Engl J Med 2008,358:261–73.
- World Health Organization (WHO). WHO case definitions for human infections with influenza A(H5N1) virus. Available at: https://www.who.int/csr/disease/avianinfluenza/guidelines/case definition2006 08 29/en/print.html Accessed on 29 August 2010.
- Schuneman H.J, Hill S.R, Kakad M, Bellamy R, Uyeki T.M, Hayden F.G. “WHO Rapid Advice Guidelines for pharmacological management of sporadic human infection with avian influenza A(H5N1) virus”, Lancet Infect Dis 2007,7:21–31.
- World Health Organization (WHO). “Antigenic and genetic characteristics of H5N1 viruses and candidate H5N1 vaccine viruses developed for potential use as pre-pandemic vaccines”. Available at: https://www.who.int/csr/disease/avian influenza/guidelines/summary H520070403.pdf. Accessed on March 2010.
- Peters C. J. “The viruses in our past, the viruses in our future” in Smith G.L, Irving W.L, McCauley J.W, Rowlands D.J. (Eds.), “New challenges to health: The threat of virus infection”, 60th Symposium of the Society for General Microbiology, Cambridge, UK, Cambridge University Press, 2001, pp: 1–32.
- Slaven E.M, Stone S.C, Lopez F.A. “Infectious Diseases. Emergency Department Diagnosis and Management”, 1st ed., chapter 37, The McGraw Hill Companies, 2006.
- Keystone J.S, Kozarsky P.E, Freedman D.O, Nothdurft D, Connor B.A. “Travel Medicine”, 1st ed., Mosby, Elsevier, 2004, pp: 1-12.
- Lashley F.R, Durham J.D. “Emerging Infectious Diseases.Trends and Issues”, 2nd ed., Springer Publishing Company, 2007, pp:1-24.
- Wilder-Smith A, Schwartz E. “Travel Medicine: tales behind the Science”, 1st ed., Elsevier Ltd, 2007, pp:1-15.
- Sarafis P, Malliarou M, Moustaka H, Gaitanou K, Zyga S, Saroglou, Manolidou Z. Understanding emerging infections: past, present and future. Balkan Military Medical Review 2008, 11(4):177-185.
- Sarafis P, Stavrakakis P, Chalaris M, Stamataki P, Zyga S, Saroglou G. Emerging infectious diseases. Journal of Environmental Protection and Ecology 2010,11(3):917-929.








