Jeevan Jyoti K1*, Yacob T1, Abdurhman N1, Asmerom S1, Birhane T1, Sebri J1 and Kaushik A2
1School of Allied Health Profession, Asmara College of Health Sciences, Asmara, Eritrea
2School of Pharmacy, Asmara College of Health Sciences, Asmara, Eritrea
*Corresponding Author:
Jeevan Jyoti K
School of Allied Health Profession, Asmara
College of Health Sciences, Asmara, Eritrea.
Tel: +291-9246374060
E-mail: jeevan_micro26@rediffmail.com
Received date: August 16, 2017; Accepted date: August 18, 2017; Published date: August 25, 2017
Citation: Jyoti K J, Yacob T, Abdurhman N, Asmerom S, Birhane T, et al. (2017) Eritrean Chewing Sticks Potential against Isolated Dental Carries Organisms from Dental Plaque. Arch Clin Microbiol. Vol. 8 No. 4:58. doi: 10.4172/1989-8436.100058
Copyright: © 2017 Jyoti K J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Traditionally used medicines; Chewing stick; Antimicrobial activity; Oral pathogens
Introduction
Dental caries is one of the most common chronic infectious diseases in the world. Bacterial plaque accumulated on dental surfaces and composed of native oral flora is the primary etiologic agent of dental carries. Dental plaque formation is the complementary process for the cause of dental carries. Therefore mechanical removal of plaque using chewing sticks is needed to inhibit the action of bacteria as well as chemically killing the bacteria and other oral pathogens responsible for dental carries [1]. Different oral hygiene methods have been in practice to defeat widely common diseases such as dental caries and oral infections. Various chemical and mechanical approaches are being used for maintaining good oral hygiene [2]. Due to Increasing awareness and expected evolving population; the use of safe, effective and economical products have expanded drastically and vary from country to country and from culture to culture [3]. Despite the widespread use of toothbrushes and toothpastes, natural methods of tooth cleaning using chewing sticks selected and prepared from the twigs, stems or roots from a variety of plant species have been practiced for thousands of years in Asia, Africa, the Middle East and the Americas [4]. School children in the mainly rural province of Matabeleland South, in Zimbabwe, were examined for dental caries and interviewed about their oral hygiene practices [5].The effect of oral hygiene programs was also studied in 248 children from five school classes in Asella, Ethiopia [6].Various clinical studies have shown that chewing sticks, when properly used, can be as efficient as toothbrushes in removing dental plaque due to the combined effect of mechanical cleaning and enhanced salivation [3]. It has also been suggested that antimicrobial substances that naturally protect plants against various invading microorganisms or other parasites may leach out into the oral cavity, and that these compounds may benefit the users by protection against cariogenic and periodontopathic bacteria [7]. A few recent studies have identified some of the active antimicrobial compounds [8]. The use of miswak becomes very popular in the Muslim world including several African and Arab countries [9]. Present study with an aim to evaluate the antimicrobial activity of ten traditionally used ten chewing sticks in Eritrea against oral pathogens isolated from dental plaque oral pathogens which are responsible for dental carries.
Materials and Methods
Plants selected for the present study
Ten different types of plants which are locally used as chewing sticks in different parts of Eritrea were collected in the month of February 2014 from different locations and were used for the present experimental study. The selected plants were authenticated by taxonomist from botany department of Eritrean Institute of Technology (EIT), Eritrea.
Preparation of aqueous extracts
The chewing sticks samples were washed under running tap water to remove dirt. The samples were shade dried for 7 days to curb distortion in the composition of the active principle in the chewing sticks [10]. The dried samples were well pulverized into a fine powder with a mixer grinder. The powder was stored in air tight aseptic containers (28ºC ± 2) for subsequent use. Weighed material (50 g each) was placed in conical flask containing 50:50 Ethanol Aqueous solution, then placed on an electronic shaker for 48 hours at the speed of 100 rpm. The fluids were then filtered using what-man number 1 filter paper. The extracts were rotary dried to obtain the concentrate which was then kept at 4ºC prior to use [11].
Plaque samples collection
Dental plaques from eight patients were picked up through forceps and periodontal probe and transferred into 5 ml of reduced transport fluid medium (0.4% agar, 0.15% thioglycollate/ phosphate buffered saline) in sterile tubes and stored at 4°C and processed within one night of collection [12]. After explanation of the purpose of the study, all subjects or their families voluntarily provided informed written consent.
Isolation of bacteria from dental plaque
Each sample in tubes was inoculated separately into nutrient agar and blood agar. Nutrient agar media is prepared by weighing 28 gram of nutrient agar powder is added to 1000 ml of distilled Water. Uniform mixing of the media is carried out by a magnetic stirrer and is heated till vapor rises. After heating, it is autoclaved at 121°C for 15 minutes and is dispensed in to a sterile Petri-plate in a biosafety cabinet. Blood agar media is prepared by adding 50 ml defibrillated (by glass beads) fresh sheep blood to the sterilized nutrient agar media just before dispensing. The inoculated plates were incubated at 37°C for 3 days under aerobic conditions. The isolated colonies were picked up and subsequently streaked on slants for pure culture preservation [13,14].
Antimicrobial study
The antimicrobial assay was performed by using the agar well diffusion method [15]. Wells of 8mm in diameter were made into previously seeded Muller Hinton agar plates. The pathogenic microorganisms used for the study were inoculated in Nutrient broth also plated in agar media and incubated for 24 hours to check the viability of the organisms. Cultures that were grown on a media were used to take 3-5 colony forming units (CFU) for preparation of suspension which was further checked for turbidity equilibration against 0.5 McFarland turbidity standards. Each well was filled with 0.14 ml of the extract. The same quantity of sterile distilled water and 50% ethanol both without plant extract served as controls. The plates were incubated overnight at 37ºC. However, same was repeated for the fungal assay. The diameter of clear zone was measured in mm. Triplicate plates were prepared for each extract and controls [16].
Results
The chewing sticks were powdered and extracted with ethanolaqueous (50: 50) solution. The extracts were concentrated under reduced pressure at 40°C. Yield percentage of different extracts was calculated and summarized in Table 1.
| S.No. |
Name of the plant |
Percentage yield ( w/w) % |
| 1. |
Cadaba farisonia |
18% |
| 2. |
Citrus lemon |
28% |
| 3. |
Dodonea angustifolia |
36% |
| 4. |
Euclea schimperi |
20.4% |
| 5. |
Grewia ferruginea |
15.2% |
| 6. |
Olea europea |
22% |
| 7. |
Parkinsonia aculeate |
10.4% |
| 8. |
Rumex nervosus |
16% |
| 9. |
Salvadora persica |
13.6% |
| 10. |
Schinus molle |
38% |
Table 1 Yield Percentage of various plant extracts.
Ten bacterial pathogens were isolated from eight patients. Cultural characteristics of isolated bacteria such as size, shape, pigmentation, elevation and margin of colony, and their biochemical properties were also evaluated.
The ethanol aqueous extract of each selected chewing stick was diluted and two different concentrations (500mg/ml and 250mg/ml) were made. These concentrations were then tested in triplicates for their antimicrobial potential against the isolated bacterial pathogens by using well as agar well method and results are tabulated in Table 2.
| Organism isolated |
Concentration of Extract |
Cadaba farinose
ZOI inmm |
Citrus lemon ZOI inmm |
Dodenia angustifolia ZOI in mm |
Euclea schimperi ZOI in mm |
Grewia ferruginea ZOIin mm |
Olea europaeZOI inmm |
Parkinsonia aculeta
ZOI in mm |
Rumex nervosus ZOIin mm |
Salvadora persica ZOI in mm |
schinus molle ZOI in mm |
| DC 1 |
500 mg/ml |
23 mm |
23 mm |
26 mm |
22 mm |
2 mm |
22 mm |
---- |
28 mm |
23 mm |
33 mm |
| |
250 mg/ml |
16 mm |
17 mm |
20 mm |
15 mm |
----- |
17 mm |
---- |
24 mm |
17 mm |
27 mm |
| DC 2 |
500 mg/ml |
24 mm |
16 mm |
19 mm |
20 mm |
3 mm |
25 mm |
3 mm |
27 mm |
15 mm |
32 mm |
| |
250 mg/ml |
25 mm |
13 mm |
14 mm |
16 mm |
1mm |
20 mm |
----- |
21 mm |
10 mm |
26 mm |
| DC 3 |
500 mg/ml |
28 mm |
19 mm |
23 mm |
22 mm |
4 mm |
27 mm |
2 mm |
26 mm |
13 mm |
29 mm |
| |
250 mg/ml |
20 mm |
13 mm |
18 mm |
16 mm |
1mm |
21 mm |
---- |
21 mm |
10 mm |
23 mm |
| DC 4 |
500 mg/ml |
27 mm |
16 mm |
20 mm |
19 mm |
5 mm |
28 mm |
4 mm |
26 mm |
14 mm |
31 mm |
| |
250 mg/ml |
22 mm |
13 mm |
16 mm |
14 mm |
2 mm |
23 mm |
---- |
21mm |
10mm |
26 |
| DC 5 |
500 mg/ml |
24 mm |
25 mm |
28 mm |
20 mm |
5 mm |
27 mm |
----- |
32 mm |
20 mm |
30 mm |
| |
250 mg/ml |
18 mm |
20 mm |
19 mm |
17 mm |
3 mm |
20 mm |
----- |
26 mm |
16 mm |
25 mm |
| DC 6 |
500 mg/ml |
24 mm |
22 mm |
25 mm |
21mm |
4 mm |
26 mm |
3 mm |
28 mm |
19 mm |
30 mm |
| |
250 mg/ml |
19 mm |
17 mm |
20 mm |
18 mm |
------ |
19 m |
----- |
22 mm |
14 mm |
24 mm |
| DC 7 |
500 mg/ml |
23 mm |
25 mm |
24 mm |
22 mm |
6 mm |
29 mm |
1 mm |
27 mm |
16 mm |
32 mm |
| |
250 mg/ml |
17 mm |
19 mm |
19 mm |
16 mm |
2 mm |
23 mm |
------ |
23 mm |
12 mm |
26 mm |
| DC 8 |
500 mg/ml |
22 mm |
24 mm |
26 mm |
22 mm |
3 mm |
28 mm |
2 mm |
29 mm |
17 mm |
31 mm |
| |
250 mg/ml |
16 mm |
18 mm |
22 mm |
16 mm |
------ |
23 mm |
------ |
25 mm |
13 mm |
27 mm |
| DC 9 |
500 mg/ml |
19 mm |
21 mm |
23 mm |
23 mm |
3 mm |
26 mm |
4 m |
28 mm |
18 mm |
30 mm |
| |
250 mg/ml |
15 mm |
17 mm |
18 mm |
18 mm |
---- |
22 mm |
------ |
23 mm |
13 mm |
25 mm |
| DC 10 |
500 mg/ml |
20 mm |
23 mm |
25 mm |
27 mm |
5 mm |
28 mm |
3 mm |
30 mm |
19mm |
32mm |
| |
250 mg/ml |
16mm |
18 mm |
20 mm |
22 mm |
2 mm |
24 mm |
------ |
26mm |
14mm |
28mm |
Table 2 Antibacterial activity of the Cold Extracts of selected chewing sticks.
Figures 1-4 indicate the antimicrobial activity against the isolated pathogens. Nearly all the extract from selected chewing sticks showed good antimicrobial activity against all the isolated pathogens except Grewia ferruginea and Parkinsonia aculeta which showed negligible zone of inhibition.
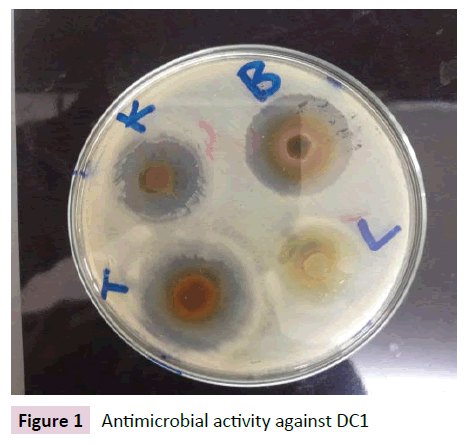
Figure 1 Antimicrobial activity against DC1
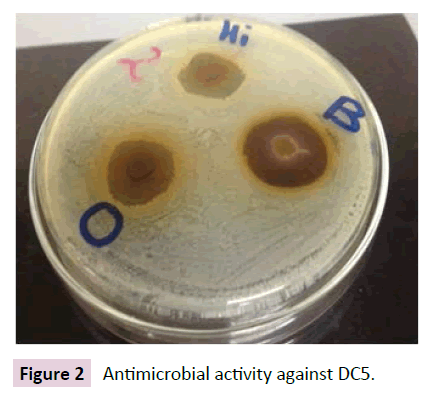
Figure 2 Antimicrobial activity against DC5.
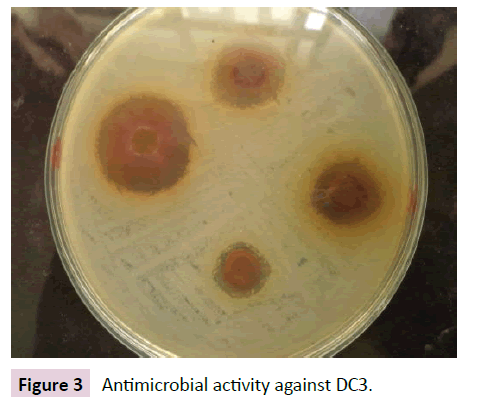
Figure 3 Antimicrobial activity against DC3.
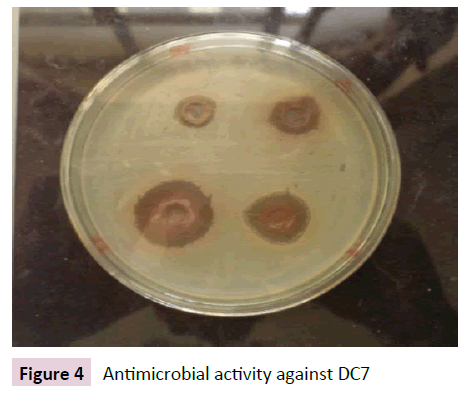
Figure 4 Antimicrobial activity against DC7
The inhibition zone size increased as the concentration of extract increased. From the result it was observed that extract of Cadaba farisonia at 500 mg/ml showed a maximum zone of inhibition of 28 mm against DC3 whereas Citrus lemon at same concentration produced a maximum zone of inhibition of 25 mm against DC5 and DC7. Extract of Dodonea angustifolia produced a maximum zone of inhibition of 28 mm against DC5 at concentration of 500mg/ml while extract of Euclea schimperi exhibited a maximum zone of inhibition (27mm) against DC10. Likewise extract of Olea europae produced a maximum zone of inhibition of 29 mm against DC7, whereas Rumex nervosus extract showed a maximum zone of inhibition of 32 mm against DC5. Cold extract of Salvadora persica and Schinus molle produced a maximum zone of inhibition of 23 mm and 33 mm respectively at 500 mg/ml against. Comparison of antimicrobial potential was performed against isolated pathogens. Among all the extracts of chewing sticks, eight chewing sticks were active against all the organisms while two sticks were showing negligible inhibition. Chewing sticks were ranked according to their antimicrobial potential. Schinus molle was most effective against nine bacterial isolates and ranked as first while Parkinsonia aculeta ranked tenth. Different chewing sticks showed different activity against selected pathogens, this may be due to various phyto-constituents present in the plant as well as their mechanism of action. All the chewing sticks were ranked from first to tenth accordingly to their activity (Tables 3 and 4). All the chewing sticks exhibited great antibacterial effects against the test organisms.
| Organism |
1st |
2nd |
3rd |
4th |
5th |
6th |
7th |
8th |
9th |
10th |
| DC 1 |
SM |
RN |
DA |
ES&SP |
OE |
CL |
CF |
GF |
PA |
- |
| DC 2 |
SM |
RN |
OE |
DA |
ES |
CF |
CL |
SP |
GF |
PA |
| DC 3 |
SM |
RN |
RN |
OE |
DA |
ES |
CL |
SP |
GF |
PA |
| DC 4 |
SM |
RN |
OE |
DA |
ES |
CL |
CF |
SP |
GF |
PA |
| DC 5 |
RN |
SM |
DA |
OE |
ES |
CF |
CL&SP |
GF |
PA |
- |
| DC 6 |
SM |
RN |
OE |
DA |
CF |
CL |
ES |
SP |
GF |
PA |
| DC 7 |
SM |
RN |
OE |
CL |
DA |
CF |
ES |
SP |
GF |
PA |
| DC 8 |
SM |
RN |
OE |
DA |
CL |
CF&ES |
SP |
GF |
PA |
|
| DC 9 |
SM |
RN |
OE |
DA |
ES |
CL |
CF |
SP |
GF |
PA |
| DC 10 |
SM |
RN |
OE |
ES |
DA |
CL |
CF |
SP |
GF |
PA |
Note: Cadaba farisonia (CF), Citrus lemon (CL), Dodonea angustifolia(DA), Euclea schimperi (ES), Grewia ferruginea (GF), Olea europea(OE), Parkinsonia aculeate(PA), Rumex nervosus (RN), Salvadora persica(SP), Schinus molle(SM).
Table 3: Ranking of each extract against bacterial isolates.
| Rank |
Plant |
| 1st |
Schinus molle |
| 2nd |
Rumex nervosus |
| 3rd |
Olea europea |
| 4th |
Dodenia angustifolia |
| 5th |
Eculea schimperi |
| 6th |
Citrus lemon |
| 7th |
Cadaba farinose |
| 8th |
Salvadora persica |
| 9th |
Grewia ferruginea |
| 10th |
Parkinsonia aculeate |
Table 4 Ranking of chewing sticks.
Note: Cadaba farisonia (CF), Citrus lemon (CL), Dodonea angustifolia(DA), Euclea schimperi (ES), Grewia ferruginea (GF), Olea europea(OE), Parkinsonia aculeate(PA), Rumex nervosus (RN), Salvadora persica(SP), Schinus molle(SM)
Discussion
The isolated bacteria were gram stained, and both gram positive and gram negative bacterial isolates were found. The shapes of bacterial cells were cocci, bacilli, single, paired, chain and dense clusters. Biochemical properties of the isolates were tested according to Bergey’s Manual of Systematic Bacteriology [17]. The properties of catalase test and acid production from carbohydrates (glucose and lactose) were determined. Haemolysis by the bacteria on blood agar media was recorded in case of isolate DC5 only. Carbohydrate fermentation test was done to identify the bacteria as acidogen. The carbohydrate fermentation test result showed that most of the isolates were highly acidic while DC3 was low and DC10 very low. Destruction of calcified tissue was caused by acids which are by product of carbohydrate metabolism of acidogenic bacteria consequent to dental caries [18].
In this study all the chewing sticks exhibited great antibacterial effects against the test organisms. Similar kind of study done by Bankole, et al. 2012 in Nigeria using two locally used chewing sticks Massularia accuminata and Distemonanthus benthamianus, both the sticks exhibited great antifungal and antibacterial effects against the test organisms. Another study performed by Odongo et al. in 2011 on eight commonly used chewing sticks by Ugandan rural community, of which R. vulgaris and L. trifolia were active against S. Mutans [19].
Astonishingly nearly 300 different species of trees and shrubs in east Africa are used in making chewing sticks. Ethnobotanical studies in Jimma, Ethiopia were carried by Kothai (2012) on eleven chewing sticks. Results revealed that Olea europaea showed maximum activity. They also evaluated the synergistic effect of chewing sticks with cinnamon and honey and observed 3 fold increases in the antimicrobial activity [20].
Conclusion
Chewing sticks are recommended for oral hygiene by the World Health Organization, and some of them, or their extracts, are also used in the ethnomedical treatment of oral infections. Chewing sticks were predominantly prepared from branches of shrubs and trees, a practice we consider bio-conservation friendly, because it is unlikely to threaten survival of the plants used. This study showed that the acidogenic bacteria mainly colonized in dental plaque cause dental caries. All the isolates were acidogens and they were sensitive against 80% of tested chewing sticks. The present finding supports the use of these chewing sticks in oral hygiene since their potential anti-plaque effect is likely to complement the mechanical plaque-removing property of chewing sticks. This is however likely to be the first time report made on the effect of sticks on oral pathogenic bacteria in Eritrea. These plants (sticks) contain compounds that are active against oral pathogens, and merit further investigation as they are possible sources of cheap dental health care for the rural poor. It is recommended that the chewing sticks will be a great help in developing countries with financial constraints and limited oral health care facilities for their populations. Hence the following recommendations can be forwarded for future research; Research for quantification of the active phytochemical compounds, purification, patenting of the active principles in the chewing sticks, preference of chewing sticks over synthetic tooth brushes because they are less likely to traumatize the gums.
Acknowledgement
Authors are highly thankful to Asmara College of Health Sciences, Asmara Eritrea for providing laboratory and chemicals support. Authors are also grateful to National Health Laboratory (NHL) for guidance in antimicrobial studies.
20106
References
- Cancro LP, Fischman SL (1995) The expected effect on oral health of dental plaque control through mechanical removal. Periodontology 8: 60-74.
- Muhammad S, Lawal MT (2010) Oral hygiene and the use of plants. Scientific Research and Essays 5: 1788-1795.
- Wu CD, Darout IA, Skaug N (2001) Chewing sticks: timeless natural toothbrushes for oral cleansing. Journal of periodontal research 36: 275-284.
- Al-Otaibi M (2004) The miswak (chewing stick) and oral health. Studies on oral hygiene practices of urban Saudi Arabians. Swedish dental journal. Supplement 167: 2-75.
- Sathananthan K, Vos T, Bango G (1996) Dental caries, fluoride levels and oral hygiene practices of school children in Matebeleland South, Zimbabwe. Community dentistry and oral epidemiology 24: 21-24.
- Olsson B (1978) Efficiency of traditional chewing sticks in oral hygiene programs among Ethiopian schoolchildren. Community dentistry and oral epidemiology 6: 105-109.
- Rotimi VO, Laughon BE, Bartlett JG, Mosadomi HA (1988) Activities of Nigerian chewing stick extracts against Bacteroides gingivalis and Bacteroides melaninogenicus. Antimicrobial agents and Chemotherapy 32: 598-600.
- Akpata ES, Akinrimisi EO (1977) Antibacterial activity of extracts from some African chewing sticks. Oral surgery, oral Medicine, oral pathology 44: 717-722.
- Niazi F, Naseem M, Khurshid Z, Zafar MS, Almas K (2016) Role of Salvadora persica chewing stick (miswak): A natural toothbrush for holistic oral health. European journal of dentistry 10: 301.
- Eloff JN (1998) Which extractant should be used for the screening and isolation of antimicrobial components from plants? Journal of ethnopharmacology 60: 1-8.
- Palombo EA, Semple SJ (2001) Antibacterial activity of traditional Australian medicinal plants. Journal of ethnopharmacology 77: 151-157.
- Hoover CI, Newbrun E (1977) Survival of bacteria from human dental plaque under various transport conditions. Journal of clinical microbiology 6: 212-218.
- Syed SA, Loesche WJ (1972) Survival of human dental plaque flora in various transport media. Applied microbiology 24: 638-644.
- McCabe RM, Keyes PH, Howell A (1967) An in vitro method for assessing the plaque forming ability of oral bacteria. Archives of oral biology 12: 1655-1656.
- Bauer AW, Kirby WM, Sherris JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. American journal of clinical pathology 45: 493.
- Bankole PO, Adekunle AA, Oyede RT, Faparusi F, Adewole A (2012) Antimicrobial activities and phytochemical screening of two tropical Nigerian chewing sticks. International Journal of Applied 2.
- Kandler O (1986) Genus Lactobacillus Beijerinck 1901, 212^< AL. Bergey's manual of systematic bacteriology 2: 1209-1234.
- Chandrabhan D, Hemlata R, Renu B, Pradeep V (2012) Isolation of dental caries bacteria from dental plaque and effect of tooth pastes on acidogenic bacteria. Open Journal of Medical Microbiology 2: 65.
- Odongo CO, Musisi NL, Waako P, Obua C (2011) Chewing-stick practices using plants with anti-streptococcal activity in a Ugandan rural community. Frontiers in pharmacology 2.
- Seshathri K (2012) Antimicrobial properties of Ethiopian chewing sticks against Candida albicans. Antimicrobial efficiency 2: 45-50









