Keywords
|
| Wet explosion; loblolly pine; Trichoderma reesei; Aspergillus saccharolyticus; Cellic®Ctec2; Novozym 188 |
INTRODUCTION
|
| Efficient conversion of lignocellulosic polysaccharides to fermentable sugars is the key for commercial production of biofuels [1,2]. Despite many technological improvements, pretreatment and utilization of commercially available lignocellulolytic enzymes to break the recalcitrant nature of lignocellulosic biomass is limiting the viability of producing biofuels [3-7]. Efficient and economically viable ethanol production requires that all sugars produced from cellulose and hemicellulose be converted to ethanol or other bioproducts [8,9]. Enzymatic hydrolysis plays a pivotal role in efficient conversion of the cellulose and hemicelluloses fractions of pretreated biomass materials. The efficiency of enzymatic hydrolysis depends on the pretreatment and right kind and proportions of enzyme cocktails that are used. |
| Numerous studies to investigate the potential of softwood conversion to ethanol have been done in the last two decades [6,10,11]. Pretreatment conditions affect the requisite enzyme mixture employed for polysaccharide hydrolysis. Previously, acid and SO2 catalyzed steam explosion was extensively used to pretreat softwood for enhancing the enzymatic hydrolysis [12- 17]. Among different pretreatment methods developed to date, wet explosion (WEx) is considered one of the most appropriate and cost effective methods for deconstruction of softwood and has demonstrated unparalleled performance for fermentable sugar production as discussed by Rana et.al. [18]. |
| In-house enzyme production has recently gaining interest for reducing the cost associated with enzymatic hydrolysis but the efficiency of those crude enzymes is limited compared to commercial enzymes. Still commercial enzymes are expensive and have been problematic for the exploitation of lignocellulosic biomass for production of biofuels [3,19]. In the present study we examine the possibility of supplementing in-house produced enzymes with small amounts of commercial enzymes to obtain sufficient hydrolytic affects with reduces dosages of commercial enzymes and thereby lowering the overall cost of enzymatic hydrolysis. |
| Fungal derived cellulolytic enzymes were investigated extensively in the last few decades for the hydrolysis of lignocelluloses [20,21]. Lignocellulolytic enzymes can be produced by a diverse group of fungi including ascomycetes (e.g. T. reesei), basidiomycetes including white-rot fungi (e.g. P. chrysosporium), brown-rot fungi (e.g. Fomitopsis palustris) and some anaerobic species (e.g. Orpinomyces sp.) which degrade cellulose in the gastrointestinal tract of ruminant animals [22]. Of these strains, aerobic fungal strains, specifically T. reesei, is of interest as it produces large amounts of extracellular cellulolytic enzymes when grown in liquid culture. Lignocellulose degradation requires three enzymatic components/domains: (endoglucanase, cellobiohydrolase and β-glucosidase), however none of the fungal strains including the best mutants described are able to produce all three required enzyme components at the same time [23]. T. reesei exhibits high Cellobiohydrolase (CBH) and Endoglucanase (EG) activities but lack sufficient β-glucosidase activities [24,25]. Therefore, in order to achieve good cellulose hydrolysis, T. reesei cellulases are typically supplemented with β-glucosidase from Aspergillus niger. |
| Fungal strains displaying cellulolytic activity are capable of degrading cellulose and have great potential to be used in a consolidated biorefinery for cost-effective biofuels production but their efficiency is generally low compared to commercial enzymes. A substantial amount of research has been done to investigate the potential benefit of accessory enzymes to supplement commercial cellulases such as commercial xylanase, arabinase, mannanase, pectinase, and other auxiliary enzymes which can reduce the amount of enzymes required for efficient biomass hydrolysis of acid or alkaline pretreated biomass. Alvira et al. [26] observed that endoxylanase and a-L-arabinofuranosidase supplementation during enzymatic hydrolysis of steam exploded wheat straw increased the enzymatic hydrolysis yield by 10%. According to Kumar et al. [27] an incremental increase in glucose release was observed with xylanase supplementation. Varnai et al. [28] observed that the addition of endo-β-mannanase increased the overall hydrolysis yield by 20-25% of cellulose. Despite a large research effort within this field there is still a lack of understanding of the effect of supplementing low-cost inhouse enzymes with small amounts of commercial enzymes for hydrolyzing pretreated biomass materials such as softwood. |
| The overall objective of this study is to evaluate the effect and potential application of supplementing in-house produced enzymes with commercial enzymes for reducing the overall cost of enzymatic hydrolysis without sacrificing the hydrolytic efficiency. The proposed research is innovative as we use hydrolysis of softwood which has proven to be more difficult than pretreating agricultural residues. In this study, sugar yields from hydrolyzing wet exploded loblolly pine under three different scenarios are compared. Scenario 1- In-house produced enzymes, Scenario 2- commercial enzymes and Scenario 3- in-house enzymes added with commercial enzymes with the objective of finding the lowest amount of commercial enzymes needed to achieve optimal saccharification yields when using in-house produced enzymes. |
Materials and Methods
|
|
Enzymes
|
| Cellulolytic enzymes produced in-house from mutant fungi, Trichoderma reesei RUT-C30, and novel fungal strain, Aspergillus saccharolyticus (CBS 127449) were studied. These fungi were used for cellulase and β-glucosidase production, respectively. Three commercial enzymes were used to evaluate the effect of supplementation or boosting. Cellic®Ctec2 and Cellic® Htec2 from Novozymes and Novozym188 from Sigma were employed. Cellic®Ctec2, Cellic® Htec2 and Novozym 188 were used as a source of cellulase, hemicellulase and β-glucosidase, respectively. Measurement of enzymatic activities was performed using different substrates. Cellulase activity was determined using filter paper (Ghose 1987) and β-glucosidase activity using pNPG (Flachner 1999) as substrates. |
| Cellulase activity (FPase) was assayed with glucose as the standard. The assay mixture comprising 0.5 mL fermentation broth sample, 1.0 mL citrate buffer (50 mM, pH 4.8) and Whatman No. 1 filter disc was incubated at 50 oC for 60 min followed by addition of 3 ml DNS reagent for color development and boiling for 5 min. Absorbance was measured at 550 nm using UV spectrophotometer. One unit of FPase activity was defined as the amount of enzyme that released 1 μmol of glucose equivalent per min per mL enzyme. |
| β-glucosidase activity was measured using 4-Nitrophenyl b-Dglucopyranoside, pNPG (Sigma Aldrich) as the substrate. 500 μl of 50 mM pNPG was mixed with 50 μl of enzyme solution in 50 mM sodium citrate buffer (pH 4.8). After 10 min of incubation at 50oC, the reaction was quenched by adding 1mL of 1 M ice-cold sodium carbonate. Subsequently, the absorbance of 4-nitrophenol produced during the reaction was measured at 405 nm using UVspectrophotometer. One unit (U) of BGL activity was defined as 1 μmol of 4-nitrophenol released per minute. |
|
Raw material and wet explosion pretreatment
|
| Representative samples of softwood (loblolly pine) chipped to approximately quarter inch were obtained from Iowa State University. The samples were screened for uniform chip size and equilibrated to a moisture content less than 10% (w/w) prior to compositional analysis and pretreatment. Wet explosion pretreatment of loblolly pine was carried out in a 10L reactor described in (Rana et al., 2012) at 170oC for 24 min and 5.5 bar oxygen. These conditions were selected according to previous optimization studies (data not published) and based on optimal sugars recovery and hydrolysis yield. |
| For analytical purposes, representative samples of the wet exploded loblolly pine slurry was filtered to obtain the solid fraction which was washed multiple times with deionized water and dried to obtain water insoluble solids (WIS). The filtered liquid was used for soluble sugars and degradation products determination. Chemical composition of both raw and pretreated solids (WIS) was determined according to Sluiter et al. [29]. The liquid fraction was hydrolyzed with 4% (v/v) H2SO4 for total sugars and sugar degradation products determination according to the standardized methods of Laboratory Analytical Procedures provided by National Renewable Energy Laboratory (NREL, Golden, CO, USA) [30]. |
|
Preparation of inoculum
|
| Inoculum cultures for fungal strain T. reesei RUT-C30 were prepared from −80?C glycerol stocks on agar plates containing 39 g L−1 yeast extract potato dextrose (YPD) and incubated static at 30?C for 120 h. The resultant spore colonies from the plates were covered with 10 mL of sterile distilled water and the suspensions were made by gently probing the surface of the plate with the tip of an inoculating loop. The composition of the medium was 30 g/l wheat bran, 25 g/l corn steep liquor (CSL), 5 g/l Avicel, 50 g/l glucose, 30 g/l peptone and 5 g/l yeast extract in a flask with a total volume of 150 ml. 50 ml of autoclaved mineral solution was added to the media with the composition: 0.3 g/l of MgSO4.7H2O, 4 g/l of KH2PO4, 2 g/l (NH4)2SO4 and 0.3 g/l of CaCl2.2H2O. Media was inoculated with 3 ml of spore’s suspension, cooled, and the cultures were grown for 48 h at 30?C in a rotary shake at 140 RPM [31]. |
| Inoculation media for A. saccharolyticus was prepared in 1000 ml shake flasks with the active volume of 200 ml. The composition of inoculation media was 40 g/l wheat bran, 40 g/l corn steep liquor (CSL), 4 g/l peptone, 2 g/l yeast extract, 2 g/l casamino acids, 12 g/l NaNO3, 1 g/l KCl, 1 g/l MgSO4 7H2O, 3 g/l KH2PO4, 0.1 g/l Na4 EDTA, 4.5 mg/l ZnSO4 7H2O, 22 mg/l H3BO3, 10 mg/l MnCl2 4H2O, 10 mg/l FeSO4 7H2O, 3.4 mg CoCl2 6H2O, 3.2 mg/l CuSO4 5H2O, 0.17 mg/l Na2MoO4 2H2O. The media was sterilized by autoclave at 121°C, for 15 min. The inoculation media was left to equilibrate in a shake incubator set to operate at 28°C, 140 RPM. After equilibration the media was inoculated using 3 ml spore suspension and the cultures were grown for 48 h at 30?C and pH 4.8 in a rotary shaker. |
|
Enzyme fermentation
|
| For enzyme production from T. reesei RUT-C30, 150 mL of the pre-culture was used to inoculate a 3 L stirred reactors. The airflow was kept at 1.2 L min−1 and the stirrer speed at 800 rpm. The culture conditions were maintained at 28?C and at pH 3.75 by automatic addition of 5 N (NH4)OH. The medium composition was as follows: 2.5% (DM) wet exploded-alkali pretreated corn stover 2.5 % corn steep liquor, 2.5% wheat bran, 0.05 % yeast extract, 0.3% peptone, 5% corn mash(liquefied with α-amylase to contain dextrin). The final volume was 1.8 L and the cells were cultured for 24 h until the ethanol was consumed (seen on the carbon dioxide drop in the off-gas), after which the corn mash (with 33% M.C.) was fed into the reactor in a fed-batch mode with an average initial dilution rate of 0.007 h-1. For saccharification of maltodextrin to D-glucose in corn mash, 300 μl of glucoamylase per liter of mash was mixed in the feed. To prevent from bacterial growth during fermentation, 0.1% v/v of kanamycin was introduced to both of the reactor and feed bottle. |
| β-glucosidase production from A. saccharolyticus was conducted in a stirred reactor (5 L) equipped with online control system for adjustment of pH, temperature, antifoam, agitation and dissolved oxygen level (DO). The fermentation was performed in a feed batch setup. 800ml of inoculation media was used as a startup media, the media was added to the 5L reactor for sterilization by autoclave, 121°C for 15 min. After sterilization the reactor was stabilized for 4 hours at 28 °C, pH 4.8, 800 RPM, aeration set-point was 0.7L air/L/m. The reactor was seeded using 200ml of inoculum as seed culture and, at 24 hours of operation the feed was started at 22 ml/h, total fermentation time was 8 days. The enzyme containing liquid was filtered and consequently concentrated 10 times by rotary vacuum evaporation. |
|
Enzymes and activities
|
| Celluclast 1.5 L and Novozyme 188, were obtained from Sigma Aldrich. Filter-paper and Carboxymethyl cellulose activities were used as a measure of cellulase activity. FPA 4.49 FPU/mL and CMCase to 20.6 U/mL was measured after 7 days of fermentation. β-glucosidase activity on pNPG was measured as 4.77 U/mL and xylanase activity as 6.61 nkat/mL. Commercial cellulase (celluclast 1.5 L) showed 81.8 FPU/mL filter paper activity, β-glucosidase activity of 58.66 U/mL and xylanase activity 107.3 nkat/mL. PNPG activities (β-glucosidase) in A. saccharolyticus and in commercial enzyme Novozym 188 were 339.9 U/mL and 698.3 U/mL, respectively. |
|
Enzymatic hydrolysis of wet exploded loblolly pine (WELP)
|
| Enzymatic hydrolysis assays were carried out in 125 mL shake flasks at 50 oC with agitation at 150 rpm at pH 5 in a shaking incubator. The total working volume was 50 mL and all the experiments were performed in quadruplicate with standard deviations less than ±2. WELP was mixed with citrate buffer (50 mM, pH 4.8) and appropriate amount of enzymes to achieve total solids 20%. All the enzymatic hydrolysis assays were conducted at 20% (w/w) total solids at the above mentioned conditions for 72 h. After 72 h, enzymatic hydrolysis was stopped. Samples were withdrawn periodically at 24, 48 and 72 h, centrifuged using bench-top centrifuge (Eppendorf, Model 5804, 8000 rpm, and 10 min) and analyzed for sugars and sugar degradation compounds. |
| Constant amounts of in-house produced cellulase (15 FPU/g cellulose from T. reesei RUT C30 and 30 CBU/g cellulose from A. saccharolyticus) was used in all the experiment with varying amounts of commercial enzymes supplementation, ranging between 1% to 100% of in-house enzymes (FPU or CBU). |
| Two controls were used, control-1, in-house produced enzymes without any supplementation with loading of 15 FPU + 30 CBU per gram cellulose and control-2 commercial enzyme, Cellic®Ctec2 with loading of 45 FPU per gram cellulose. 45 FPU/g cellulose of Cellic®Ctec2 represents the sum of both filter paper as well as β-glucosidase activities. For the supplementation assays, commercial cellulase, hemicellulase and β-glucosidase were used. Cellic®Ctec2, Cellic®Htec2 were used in a dosage ranging from 1% to 100% of the total FPU (15 FPU) of the in-house enzymes. Novozym 188 was used with dosage ranging from 1% to 100% of total CBU (30 CBU) of in-house enzymes. |
|
Analytical methods and calculations
|
| Sugars concentration were measured by high-performance liquid chromatography using Bio-Rad (Hercules, CA, USA) Aminex HPX 87P column with RI detector, operating at 83oC with a flow rate of 1.0 ml/min with Milli-Q water (Barnstead Nanopure, USA) as mobile-phase. |
| Sugars degradation compounds (furfural, 5-hydroxymethylfurfural (HMF) and acetic acid) and ethanol were analyzed using an Aminex ion exclusion HPX-87H cation-exchange column (Bio- Rad, Hercules, CA) at 60oC equipped with 1050 photodiode-array detector (Agilent Technologies, Santa Clara, CA). 89% 5 mM/L H2SO4 and 11% acetonitrile were used as mobile phase at a flow rate of 0.6 mL/min were used. |
| Enzymatic hydrolysis yields were calculated based on the amount of the reducing sugars released in the hydrolysate divided by the respective carbohydrate present in raw loblolly pine multiplied with correction factor of 0.9 for hexose and 0.88 for pentoses (to correct the increased weight from hydrolysis), as shown in the following equation: |
| Enzymatic hydrolysis yield%=(Reducing sugar (g) × 0.9 (or 0.88) × 100)/Polysaccharide (g) |
| Mean values and standard deviations were calculated from quadruplicates. |
Results and Discussion
|
|
Enzyme activities
|
| Cellulase preparations from in-house produced enzymes preparations were characterized by measuring different enzymatic activities. Filter paper activity of 4.49 FPU/mL and activity on PNPG (as a measure of β-glucosidase activity) was measured as 4.77 U/mL from cellulase produced in-house from T. reesei RUT C30. A. saccharolyticus presented practically no FPA activity and 339.9 U/mL of β-glucosidase activity. It was observed that T. reesei RUT C30 exhibited insignificant β-glucosidase activity and that A. saccharolyticus has low filter paper activity. |
| Higher cellulases (FPU) were detected in Cellic®Ctec 2 and Cellic®Htec2, 100 and 94.3 FPU/mL, respectively. β-glucosidase activity in these enzymes were 396.3 U/mL and 312.1 U/mL, respectively. Higher b-glucosidase activity was detected in Novozym 188, 698.3 U/mL |
|
Raw material and Wet explosion pretreatment of loblolly pine
|
| Table 1 shows the composition of WIS and liquid fractions. Wet explosion pretreatment resulted in solubilization of hemicellulose into the liquid stream and a higher proportion of cellulose (47.2%) was left in pretreated WIS. 2.4 g/L glucose and 8.2 g/L xylose were recovered in the liquid fraction; however some sugars were degraded to HMF (0.7 g/L, derived from hexoses) and furfural (1.3 g/L, derived from pentoses). Acetic acid (3.6 g/L) was released from acetyl groups contained in the hemicellulose. |
| Some authors suggest inhibitors released during pretreatment could have a negative effect on the enzymatic hydrolysis and subsequent fermentation thus washing after pretreatment is preferred [32-36]. However, considering the capital cost of filtration and washing and losses of potential fermentable sugars due to washing, utilization of the whole slurry for the enzymatic hydrolysis may be more practical. Our results further show that very low concentrations of sugar degradation products were produced, therefore we used whole slurry after wet explosion pretreatment for the enzymatic hydrolysis to increase the concentration of fermentable sugars. Higher concentrations of sugars result in higher ethanol concentration in the subsequent fermentation step which reduces the downstream distillation and concentration costs [37,38]. |
|
Enzymatic hydrolysis
|
|
Effect of Cellic®Ctec2 supplementation on in-house produced enzymes for WELP hydrolysis
|
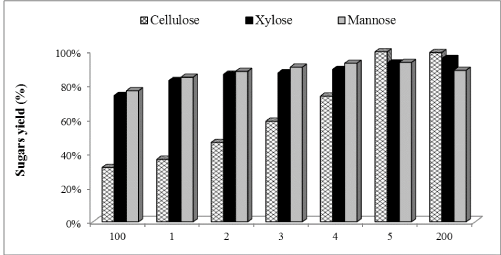
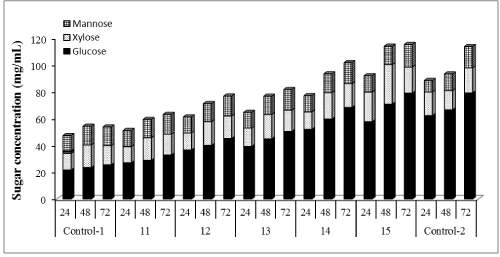
| The in-house produced enzymatic preparation was supplemented with Cellic®Ctec2 in amounts ranging between 1% and 100% of total FPU/g cellulose of in-house enzyme preparation used. Figures 1-3 show the glucose, xylose and mannose production during 72 h of enzymatic hydrolysis with boosting from different commercial enzymes (Cellic®Ctec2) for hydrolyzing WELP. It was observed that supplementation with commercial Cellic®Ctec2 improved the sugars released after 72 h hydrolysis compared to the control mixture (Figure 1). The highest enzymatic hydrolysis yields were achieved with 100% supplementation; interestingly, increasing the supplementation beyond 30% only led to modest increases in yields. Supplementation with commercial cellulase not only improved cellulose conversion but also hemicellulose conversion, which demonstrates the ability of the commercial enzyme in improving the overall hydrolysis performance. |
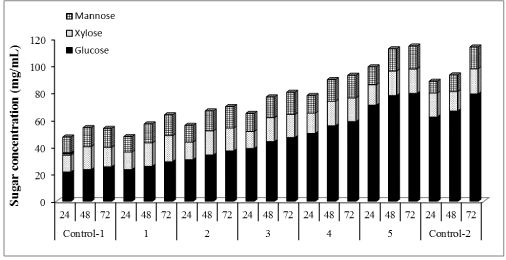
| Concentrations of glucose, xylose, and mannose were determined to be 25.39, 14.33, 13.87 mg/mL (Figures 4-6) corresponding to 32%, 74% and 77% yields, respectively using our nonsupplemented enzyme in-house enzyme cocktail (control-1). At 1% supplementation a slight change in yield was observed compared to control-1, giving an increase in glucose, xylose and mannose concentrations of 13%, 11% and 9%, respectively. At 10% supplementation, the glucose, xylose and mannose concentration increased by 32%, 14% and 13%, respectively after 72 h and the corresponding increases for 30% addition were 46%, 15% and 15% at 30%, respectively. Only small increases in the sugar release was found between 30% and 50% supplementation as shown in Figure 1, demonstrating an increase of 57%, 17% and 17% in glucose, xylose and mannose yields, respectively. At 100% supplementation the highest sugars yields were found giving 68%, 21% and 18%, of glucose, xylose and mannose, respectively. In general, Cellic®Ctec2 (cellulases) supplementation resulted in higher production of glucose compared to hemicellulose. These results were statistically supported by Tukey HSD analysis with 95% confidence level (data not shown). |
| Cellic®Ctec2 is produced by Trichoderma spp., which secretes an array of lignocellulolytic enzymes including cellulases, xylanases, abrabinosidases and β-xylosidase [39]. Mainly they exhibit cellulase activity while other enzymes are expressed at low levels. Our results clearly show that high cellulase activity improves the enzymatic hydrolysis yields during hydrolysis of pretreated loblolly pine. |
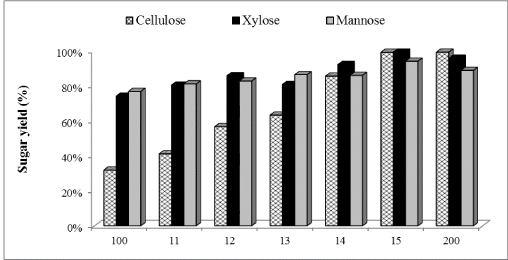
| Effect of Cellic®Htec2 supplementation on in-house produced enzymes for WELP hydrolysis |
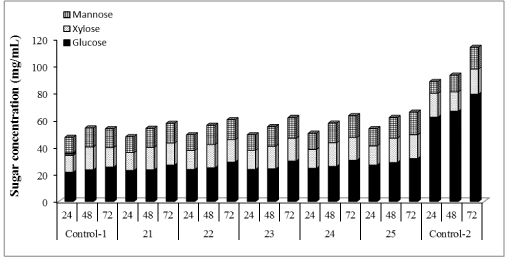
| Cellic®Htec2 (1% to 100%) was supplemented with our in-house preparation: cellulases (15 FPU/g cellulose) and β-glucosidase (30 CBU/g cellulose) and results shown in Figure 2 demonstrated that hemicellulase supplementation improved the enzymatic hydrolysis yield of WELP. Glucose production increased by 23%, 44%, 50%, 63% and, 68% with 1, 10, 30, 50 and 100% supplementation of in-house enzymes with Cellic®Htec2 respectively, compared to the non-supplemented control. For xylose production, supplementation resulted in an increase of 8%, 14%, 8%, 20% and, 25% with 1, 10, 30, 50 and 100% supplementation, respectively, compared to the non-supplemented control. Mannose production increased by 5%, 7%, 11%, 11% and, 18% with 1, 10, 30, 50 and 100% supplementation, respectively, compared to nonsupplemented control. Cellic®Htec2 supplementation resulted in higher concentration of glucose, xylose and mannose compared to the non-supplemented control, and these differences were significant at 95% confidence level of statistical analysis. With lower level of supplementation, lower yields were obtained; this clearly shows that the recalcitrance of loblolly pine unlike agricultural biomass such as corn stover demands a very active enzyme cocktail for good hydrolysis of pretreated softwood [40]. |
| The hemicellulose degradation due to synergistic effect of xylanases, arabinases and mannanase prevents the blocking effect of pentose sugars. Arabinan supposedly, blocks the access to β-1,4 xylan chain for xylanases [41]. Furthermore, xylooligomers has previously been found to have an inhibitory effect on cellulase action [27,42,43]. Therefore, removal of oligomers could improve production of xylose, arabinose and mannose and also minimize the inhibitory action on cellulase. It has been observed that supplementation of cellulases with xylanases enhances the glucose release from pretreated biomass solids [44-46]. Moreover, xylanase supplementation is more efficient when xylan is present in lower amounts in pretreated material [45] which is true for the present study. Overall, our results would suggest that supplementation of the cellulases with hemicellulase has a great impact on enzymatic hydrolysis yields and process efficiency. |
|
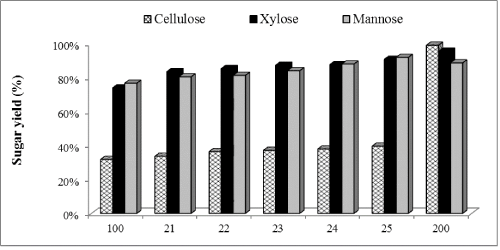
Effect of Novozym 188 supplementation on inhouse produced enzymes for WELP hydrolysis
|
| Figure 3 shows glucose, xylose and mannose yields after enzymatic hydrolysis of WELP supplemented with Novozym 188. Novozym 188 contains high amounts of β-glucosidase and helps in cellobiose conversion to glucose and also prevents endproduct inhibition of cellulase due to accumulation of cellobiose. After 72 h, glucose yield increased by 6%, 13%, 14%, 16% and, 20% ; xylose yield increased by 5%, 6%, 9%, 13% and, 17% and mannose yield increased by 2%, 3%, 6%, 10% and, 14% with 1, 10, 30, 50 and 100% supplementation, respectively, compared to the non-supplemented control. Glucose increase was supported statistically at the 95% confidence level. Less difference was observed in xylose and mannose production. Novozym 188 supplementation resulted in slightly higher concentration of xylose and mannose compared to the non-supplemented control, however, these differences were not significant at the 95% confidence level of statistical analysis. |
| A parallel study of enzymatic hydrolysis with only commercial cellulases, Cellic®Ctec2 (Control-2) was conducted. An enzyme loading of 45 FPU/mL was used for hydrolysis. Glucose, xylose and mannose concentrations after 72 h of hydrolysis were 79.1, 18.5 and 16.0 mg/mL corresponding to 99%, 96% and 89% yields, respectively. Comparing the yields control-1 (in-house enzymes without any supplementation) and control-2 (commercial enzyme only), we found that in-house enzymes supplemented with commercial enzymes resulted in higher conversion of polysaccharides after wet explosion pretreatment of loblolly pine. 15FPU/mL in-house enzymes boosted with 15FPU/mL commercial cellulase, Cellic®Ctec2 resulted in 99%, 93% and 93% of glucose, xylose and mannose yields, respectively. When 15FPU/ mL in-house enzymes was boosted with 15FPU/mL commercial hemicellulase, Cellic®Htec2, glucose, xylose and mannose yields reached to 99%, 99% and 94%, respectively. Similar cellulose conversion was achieved with Cellic®Ctec2 and Cellic®Htec2 as can be explained by similar FPA activities (100 and 94.3 FPU/mL, respectively), but higher yields of xylose and mannose was found with hemicellulase, Cellic®Htec2. |
| Table 2 summarizes cellulose conversions from hydrolysis of WELP. Cellulose conversion was increased with increasing supplementation of all three commercial enzymes. Probably due to insufficient hemicellulolytic activity, higher accumulation of hemicellulosic oligomers was observed with non-supplemented enzyme preparations. |
| The best enzymatic hydrolysis yields were obtained when the in-house cellulase cocktail was supplemented with Cellic®Htec2. When 15 FPU/mL of in-house cellulase was boosted with 7.5 FPU/mL of commercial hemicellulase, Cellic®Htec2 (50% supplementation), 85% glucose, 92% xylose and 86% mannose were produced. Contrary to some previous studies [15,47,48], higher yields (>70%) of glucose can be obtained with high loadings (30-80 FPU/g cellulose) of commercial enzymes. We obtained higher glucose yield (85%) with lower FPU/g cellulose. Increasing the supplementation from 50% to 100% resulted in a near complete sugars conversion. Furthermore, the enzymes will remain functional during fermentation and therefore able to convert remaining sugars to monomers along with the fermentation which means that any remaining cellulose can be converted to glucose during subsequent fermentation step [49]. |
| 1% supplementation of in-house enzymes with Cellic®Ctec2, Cellic®Htec2 and Novozym 188 showed glucose yields of 37%, 41% and 34% corresponding to increase in glucose yield from non-supplemented control-1(in-house enzymes) as 13%, 23% and 6% respectively. 100% supplementation of in-house enzymes with Cellic®Ctec2, Cellic®Htec2 and Novozym 188 resulted into glucose yield of 99%, 99% and 40% respectively. At highest supplementation (100%), Cellic®Ctec2 and Cellic®Htec2 resulted into similar yield of 99%, and that could be explained as, Cellic®Ctec2 primarily focuses on cellulose degradation while Cellic®Htec2 degrades hemicellulose and thus releases cellulose for cellulase attack. Also, higher enzymes/substrate ratio eliminates enzyme inhibition due to substrate characteristics. 50% supplementation, of in-house enzymes with Cellic®Ctec2, Cellic®Htec2 and Novozym 188 resulted in glucose yield of 74%, 85% and 38%. |
| Considering the amount of enzymes used and yield, 50% supplementation (7.5 FPU) with Cellic®Htec2 to in-house produced enzymes cocktail (15 FPU) was determined as optimal. Thus it is imperative that in-house produced cellulases from mesophilic fungi and commercial available cellulase formulations can be made to work synergistically as a lignocellulolytic enzyme system, providing better saccharification yields from lignocellulosic biomass for a lower cost than using expensive commercial enzymes alone. It is important to note that although the focus in this study was on monomeric sugars release enzymatic hydrolysis for longer period (more than 72 h) will likely result in higher sugar yields. Further studies are needed to evaluate the effect of other important factors such as pH, temperature and hydrolysis period on the efficiency of mixed enzymes cocktail. |
Conclusion
|
| The supplementation of cellulase mixtures produced in-house with commercial enzymes was shown to have potential for improving the enzymatic hydrolysis of softwood. This study indicated that wet explosion pretreatment can provide good raw material for enzymatic hydrolysis with in-house produced cellulases (T. reesei RUT C30 and A. saccharolyticus), however, for saccharification at high solids loading of recalcitrant biomasses such as softwood, supplementation with commercial enzymes can be advantageous. Supplementation with commercial enzymes and in particular the hemicellulase, Cellic® Htec2 significantly improved the sugar release by enzymatic hydrolysis. Higher hydrolysis yields (glucose, 85%; xylose, 92%; mannose, 86%) were achieved when in-house cellulase was supplemented with commercial hemicellulase, Cellic®Htec2 in a ratio of 15FPU:7.5FPU per mL. Our findings show ways for potentially lowering the cost of enzyme hydrolysis in biorefinery. However, further studies are needed to fully exploit the use of in-house enzymes supplemented with commercial enzymes. |
Acknowledgement
|
| National Advanced Biofuel Consortium (NABC) and Department of Energy, USA Grant ZFT04064401 are gratefully acknowledged for the financial support. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|











