Key words
|
| |
| Anti-diarrhoeal effect, Terminalia belerica, Castor oil induced diarrhoea, PGE2 induced enteropooling, Gastrointestinal motility test |
| |
Introduction
|
| |
| In developing country, the majority of people living in rural areas are using traditional medicines in treating all sorts of diseases including diarrhoea. There are large numbers of epidemiological & experimental evidence pertaining to worldwide acute diarrhoeal diseases that are one of the principal cause of death in infants, particularly in developing countries [1]. Diarrhoea (Greek and Latin: dia, through, and rheein, to flow or run) is the passage of loose or watery stools, usually at least three times in a 24-hour period. Scientists usually define diarrhoea as excessive fluid weight with 200g per day representing the upper limit of normal stool water weight for healthy adults in the western world [2]. There are an estimated 1.8 billion episodes of childhood diarrhoea per year and virtually all of these acute diarrhoeal episodes are related to infectious agents. In some parts of Africa preschool children may suffer up to seven attacks of acute diarrhoea annually, although the average worldwide is approximately three episodes per year [3]. Diarrhoea can be regarded as a global menace which can culminate in mortality and morbidity of the incumbent due to loss of fluids and electrolytes from the body. The plausible implicating factors inducing diarrhoea can range from infective to immunological and nutritive factors [4]. World-wide, nearly nine million children (under the age of five years) die every year as a result of diarrhoea. An estimated 88% of diarrhoeal-related deaths are caused by inadequate sanitation and poor hygiene. Diarrhoeal diseases are one of the leading causes of childhood morbidity and mortality in developing countries [5]. |
| |
| Terminalia belerica (Family: Combretaceae) is also known as Vibhitaka, Aksha, Vipitakaha (Sanskrit), Bahera, Bhaira (Hindi), Berang (Gujarati). This tree is an important ayurvedic herb and is found throughout India, Burma & Ceylon below elevations of about 3000 ft., except in dry & acrid region of Sind & Rajputana [6]. The fruits contain about 20 to 30% of tannins, fixed oil, saponins, a resinous residue and three amorphous, hygroscopic glycosidal compounds and colouring matter. The fruit of this plant possesses antibacterial properties. It is employed in dropsy, piles and diarrhoea, fruits is bitter, astringent, laxative, analgesics and brain tonic [7], antipyretic, useful in the dyspepsia, diarrhoea, bilious headache, eyes, to piles, brain tonic (unani) [6]. Since, there is no scientific study to substantiate the traditional claim on anti-diarrhoeal activity of the plant, in view of this, the present study was undertaken to evaluate anti-diarrhoeal activity of fruit pulp extracts of Terminalia belerica using various experimental diarrhoeal models in rats. |
| |
MATERIALS AND METHODS
|
| |
|
Plant collection and authentication
|
| |
| Fruit of Terminalia belerica were collected from the local market of Sitamarhi district of Bihar. The authentication was done by Dr. Shiddamallaya N. of Regional Research Institute (Ay.), Bangalore, Karnataka. A voucher specimen No. was RRI/BNG/SMP/drug authentication/2007-08-284. |
| |
|
Preparation of Extract
|
| |
| The fruit pulp was collected and shade dried. The fruit pulp was coarsely powdered and packed in to Soxhlet column and extracted with ethanol at 77-79ºC, and distilled water at 100ºC. The extracts were concentrated by distillation then dried on water bath. The dried extracts were stored in airtight container. |
| |
| Phytochemical evaluation: Phytochemical examinations were carried out for all the extracts as per the standard methods. |
| |
| Detection of alkaloids: Extracts were dissolved individually in dilute Hydrochloric acid and filtered. |
| |
| a) Mayer’s Test: Filtrates were treated with Mayer’s reagent (Potassium Mercuric iodide). Formation of a yellow cream precipitate indicates the presence of Alkaloids. |
| |
| b) Wagner’s test: Filtrates were treated with Wagner’s reagent (Iodine in potassium iodide). Formation of brown/reddish precipitate indicates the presence of alkaloids. |
| |
| c) Dragendroff’s test: Filtrates were treated with Dragendroff’s reagent (solution of potassium bismuth iodide). Formation of red precipitate indicates the presence of alkaloids. |
| |
| d) Hager’s test: Filtrates were treated with Hager’s reagent (saturated picric acid solution). Presence of alkaloids confirmed by the formation of yellow colored precipitate. |
| |
| Detection of carbohydrates: Extracts were dissolved individually in 5 ml distilled water and filtered. The filtrates were used to test for the presence of carbohydrates. |
| |
| a) Molisch’s Test: Filtrates were treated with 2 drops of alcoholic α-naphthol solution in a test tube and 2 ml of Conc. Sulphuric acid was added carefully along the sides of the test tube. Violet ring at the junction indicates the presence of Carbohydrates. |
| |
| b) Benedict’s test: Filtrates were treated with Benedict’s reagent and heated on water bath. Orange red precipitate indicates the presence of reducing sugars. |
| |
| c) Fehling’s test: Filtrates were hydrolysed with dil. HCl, neutralized with alkali and heated with Fehling’s A & B solutions. Formation of red precipitate indicates the presence of reducing sugars. |
| |
| Detection of glycosides: Extracts were hydrolysed with dil. HCl, and then subjected to test for glycosides. |
| |
| a) Modified Borntrager’s Test: Extracts were treated with Ferric Chloride solution and immersed in boiling water for about 5 minutes. The mixture was cooled and shaken with an equal volume of benzene. The benzene layer was separated and treated with ammonia solution. Formation of rose-pink colour in the ammonical layer indicates the presence of anthranol glycosides. |
| |
| b) Legal’s test: Extracts were treated with sodium nitropruside in pyridine and methanolic alkali. Formation of pink to blood red colour indicates the presence of cardiac glycosides. |
| |
|
Detection of saponins
|
| |
| a) Froth Test: Extracts were diluted with distilled water to 20ml and this was shaken in a graduated cylinder for 15 minutes. Formation of 1 cm layer of foam indicates the presence of saponins. |
| |
| b) Foam test: Small amount of extract was shaken with little quantity of water. If foam produced persists for ten minutes it indicates the presence of saponins. |
| |
|
Detection of phytosterols
|
| |
| a) Salkowski’s Test: Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of Conc. Sulphuric acid, shaken and allowed to stand. Appearance of golden yellow colour indicates the presence of triterpenes. |
| |
| b) Libermann Burchard’s test: Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of acetic anhydride, boiled and cooled. Conc. Sulphuric acid was added carefully along the sides of the test tube. Formation of brown ring at the junction indicates the presence of phytosterols. |
| |
|
Detection of phenols
|
| |
| Ferric Chloride Test: Extracts were treated with few drops of ferric chloride solution. Formation of bluish black colour indicates the presence of phenols. |
| |
|
Detection of tannins
|
| |
| Gelatin Test: To the extract, 1% gelatin solution containing sodium chloride was added. Formation of white precipitate indicates the presence of tannins. |
| |
|
Detection of flavanoids
|
| |
| a) Alkaline Reagent Test: Extracts were treated with few drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on addition of dilute acid, indicates the presence of flavonoids. |
| |
| b) Lead acetate Test: Extracts were treated with few drops of lead acetate solution. Formation of yellow colour precipitate indicates the presence of flavonoids. |
| |
|
Detection of proteins and aminoacids
|
| |
| a) Xanthoproteic Test: The extracts were treated with few drops of concentrated Nitric acid solution. Formation of yellow colour indicates the presence of proteins. |
| |
| b) Ninhydrin test: To the extract, 0.25% ninhydrin reagent was added and boiled for few minutes. Formation of blue colour indicates the presence of amino acid. |
| |
|
Detection of diterpenes
|
| |
| Copper acetate Test: Extracts were dissolved in water and treated with few drops of copper acetate solution. Formation of emerald green colour indicates the presence of diterpenes [8]. |
| |
|
Animals used
|
| |
| Albino Rats (Wistar strain) weighing 150-200 gm of either sex were used. They were procured from M/s. Veterinary College, Bangalore. The animals were allowed for acclimatization for ten days under laboratory conditions. They were housed in polypropylene cages and maintained at 27ºC±2ºC, relative humidity 65±10% under 12 hrs light / dark cycles. The animals were fed regularly and water ad libitum. Protocol was approved from the Institutional Animal Ethics Committee (IAEC) its reference no was IAEC/PP/03/2006-2007. |
| |
|
Anti-diarrhoeal activity
|
| |
|
Castor oil induced diarrhoea
|
| |
| Healthy albino rats of the either sex (160-190 g) were divided into 8 groups of 6 animals each. They were fasted for 18 h prior to the test, with free access to water. Group I received the vehicle and served as the control group. Groups II was treated with standard drug (Loperamide 3 mg/kg), Group III, IV &V were treated with 334 mg/kg, 200 mg/kg, 143 mg/kg doses of AQETB and group VI, VII & VIII received 334 mg/kg, 200 mg/kg, 143 mg/kg doses of EQETB respectively. All drugs/vehicle were administered orally (p.o.). After thirty minutes of the drug treatment, each rat was administered 1 ml of castor oil orally and housed separately in plastic cages, with special provision to collect faecal matter on filter paper. The diarrhoeal episodes were observed for 4 h. The cumulative wet weight of the stools was noted at the end of the 4th h. Percent inhibition of diarrhoea was calculated using the mean stool weight. Anti-diarrhoeal activity was determined in terms of percentage protection. |
| |
|
Prostaglandin-E2 induced enteropooling
|
| |
| Eight groups of albino rats of either sex, each comprising 6 animals (160-200 g), were fasted for 18 h prior to the experiment. Group I was treated with vehicle which served as control. Group II was administered with standard (Loperamide 3 mg/kg p.o.), Group III, IV &V were treated with 334 mg/kg, 200 mg/kg, 143 mg/kg doses of AQETB and group VI, VII & VIII received 334 mg/kg, 200 mg/kg, 143 mg/kg of EQETB respectively. Immediately after that, all the rats were treated orally with prostaglandin E2 (100 ìg/kg) in 5% ethanol in normal saline, and were sacrificed after 30 min. The intestine from the pylorus to the caecum was dissected and the intestinal contents were measured. Percentage reduction of intestinal secretion (volume) was calculated. |
| |
|
Gastrointestinal motility test
|
| |
| Albino rats of either sex (160-200 g) were divided into 8 groups of 6 animals each. They were fasted for 24 h prior to the test, but allowed free access to water. Group I was treated with vehicle (p.o.), which served as control, Group II treated with standard drug atropine sulphate (5 mg/kg, p.o.), Group III, IV and V administered with 334 mg/kg, 200 mg/kg, 143 mg/kg of AQETB (p.o.), Group VI, VII and VIII treated with 334 mg/kg, 200 mg/kg, 143 mg/kg of ETETB respectively. After 30 min, 1 ml of charcoal (3% deactivated charcoal in normal saline) was administered orally to all rats and 30 min later, all the rats were sacrificed. The distance travelled by the charcoal from the pylorus to the caecum was noted. |
| |
|
Statistical evaluation
|
| |
| The results of each groups were subjected to one-way analysis of variance (ANOVA) followed by Dunnett's test and P <0.05 was considered significant. |
| |
RESULTS
|
| |
|
Phytochemical screening
|
| |
| The analysis revealed that the presence of carbohydrates, fats and oils, glycosides, flavonoids, alkaloid, tannins, saponins in the aqueous and ethanolic extracts of fruit pulp of Terminalia belerica. (Table 1) |
| |
|
Castor oil induced diarrhoea
|
| |
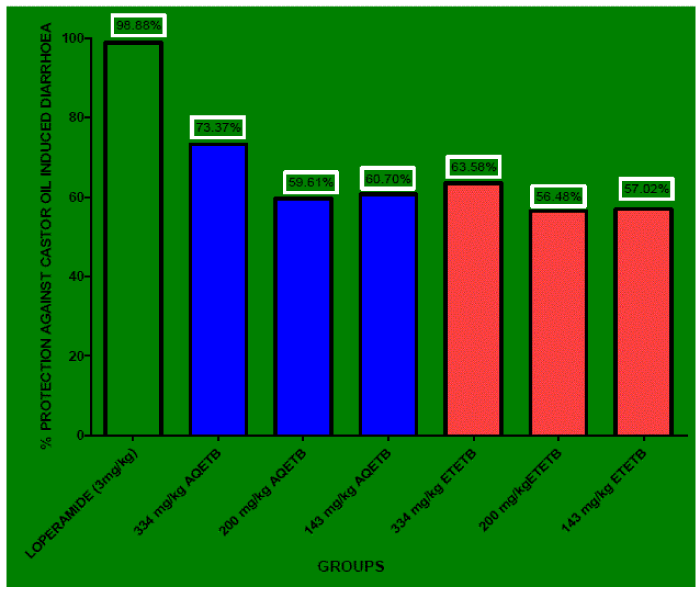
| All the doses of AQETB tested exhibited very significant anti-diarrhoeal effect on oral administration against castor oil induced diarrhoea (Table 2). The AQETB prolonged the onset time of diarrhoea (i.e. delayed the onset time of defecation) 137.5min, 135min, and 65 min. with the doses of 334 mg/kg, 200 mg/kg, and 143 mg/kg of AQETB respectively. There was very significant (p<0.01) delay in the onset of diarrhoea with 334 mg/kg of AQETB, 200 mg/kg of AQETB but 143 mg/kg AQETB showed nonsignificant (p>0.05) effect in delay in onset of diarrhoea. AQETB also decreased the no. of wet defecation, no. of dry defecation and paper weight (in gm) with respect to control and their effect was very significant (p<0.01). The percentage protection against castor oil induced diarrhoea by 334 mg/kg, 200 mg/kg, 143 mg/kg of AQETB with respect to control was found as 73.37, 59.61 and 60.70 respectively. On the other hand 334 mg/kg, 200 mg/kg, 143 mg/kg of ETETB also showed delay in the defecation time i.e. 131.6min, 123.33min, 79.16min respectively. The delay in the defecation time by 334mg/kg of ETETB was very significant (p<0.01), by 200 mg/kg of ETETB was significant (p<0.05) and 143 mg/kg of ETETB was non-significant (p>0.05). All the doses of ETETB also decreased the no. of wet defecation, no. of dry defecation and paper weight (in gm) with respect to control and the value was very significant (p<0.01). The percentage protection against castor oil induced diarrhoea by 334 mg/kg, 200 mg/kg, 143 mg/kg of ETETB with respect to control was found as 63.58, 56.58 and 57.02 respectively. 334 mg/kg of both the extracts showed higher percentage protection but 200 mg/kg, 143 mg/kg of both the extract showed nearly equal percentage protection ranges from 57 to 60 (Figure 1). AQETB showed more percentage protection with respect to ETETB when compared with control. Overall the percentage protection given by loperamide (3mg/kg), 334 mg/kg of AQETB, and 334 mg/kg of ETETB was obtained as 98.88, 73.37, and 63.58 respectively. |
| |
|
PGE2 induced enteropooling
|
| |
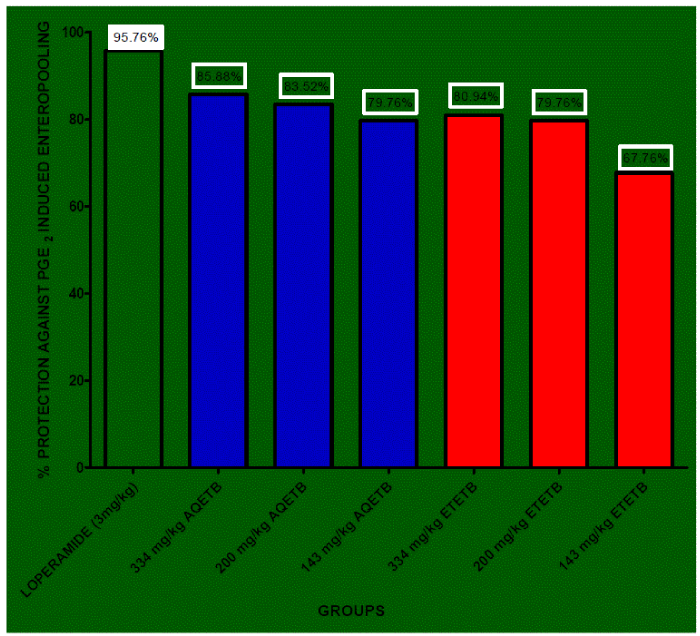
| PGE2 significantly increased the fluid volume in rat intestine in control group (Table 3). The percentage reduction of enteropooling exhibited by 334 mg/kg, 200 mg/kg, 143 mg/kg of AQETB was 85.88, 83.52, 79.76 respectively with respect to control and the reduced volume of intestinal content by AQETB was very significant (p<0.01). On the other hand 334 mg/kg, 200 mg/kg, 143 mg/kg of ETETB showed very significant (p<0.01) reduction in the volume of intestinal content and their percentage protection against enteropooling was 80.94, 79.76 and 67.76 respectively. The results indicate though there is dose dependent anti-enteropooling activity by both the extracts but there is no much increase in the effect by the doses of 200 mg/kg and 300 mg/kg (Figure 2). Anti-enteropooling effect of AQETB was more than ETETB. |
| |
|
Gastrointestinal motility test
|
| |
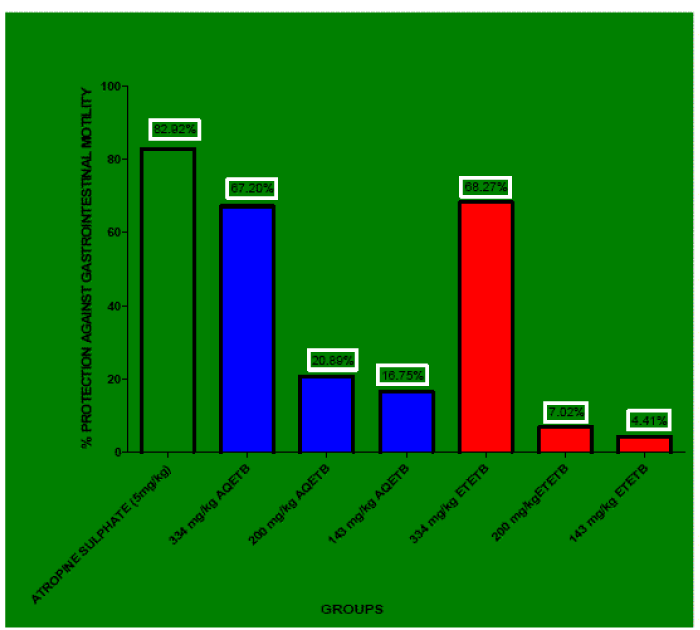
| AQETB and ETETB decreased significantly the propulsion of charcoal meal through the gastrointestinal tract when compared with control (Table 4). Percentage inhibition of charcoal movement in GIT by 334 mg/kg, 200 mg/kg, and 143 mg/kg of AQETB was 67.20, 20.89, and 16.75 respectively. AQETB at the dose of 334 mg/kg showed the very significant (p<0.01) percentage movement of charcoal from pylorus to caecum. In case of 200 mg/kg, 143 mg/kg of AQETB percentage movement of charcoal from pylorus to caecum was non- significant (p>0.05). On the other hand 334 mg/kg, 200 mg/kg, 143 mg/kg of ETETB showed percentage reduction of charcoal movement in GIT was 68.27, 7.02, and 4.41 respectively. ETETB at the dose of 334 mg/kg showed the very significant (p<0.01) percentage movement of charcoal from pylorus to caecum. In case of 200 mg/kg, 143 mg/kg of ETETB percentage movement of charcoal from pylorus to caecum was non-significant (p>0.05). Mainly 334 mg/kg of AQETB and 334 mg/kg of ETETB showed 67.20 and 68.27 percentage reduction in the movement of charcoal in GIT with respect to control and atropine sulphate 5 mg/kg exhibited 82.92 of reduction of charcoal movement (Figure 3). At the dose of 334 mg/kg of both extracts showed almost equal value of percentage protection of GI motility i.e. 67.20 and 68.27 respectively. |
| |
DISCUSSION AND CONCLUSION
|
| |
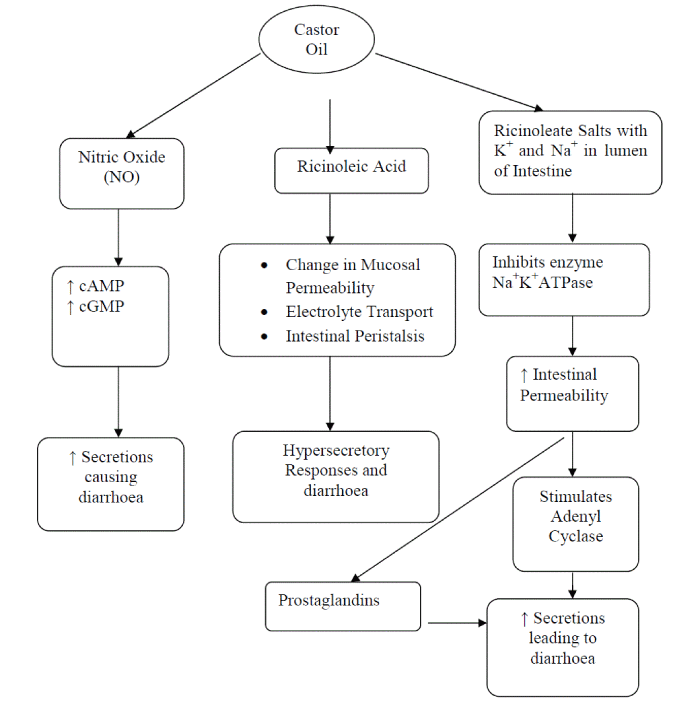
| Many anti-diarrhoeal agents act by reducing the gastrointestinal motility and/or the secretions. It is well known that ricinoleic acid, an active compound of castor oil formed in the upper small intestine and being polar poorly absorbed [1,9] induces changes in the mucosal permeability, electrolyte transport and intestinal peristalsis, leading to hypersecretory response and diarrhoea. The ricinoleic acid readily forms ricinoleate salts with sodium and potassium in the lumen of the intestine. Ricinoleate has several actions that could account for its anti-absorptive effect on the mucosa. It inhibits the enzyme sodium-potassium ATPase and increases the permeability of the intestinal epithelium, produces a cytotoxic effect on isolated enterocytes. It may stimulate epithelial cell adenyl cyclase, releases PG or other metabolite of arachidonic acid and platelet activating factor [10, 11]. Ricinoleate acts as a calcium ionophore, increasing the influx of extracellular calcium which activates calmodulin dependent secretory mechanism [11, 12]. Ricinoleic acid causes local irritation and inflammation of the intestinal mucosa leading to PG release, which causes an increase in the net secretion of water and electrolytes into the small intestine. Inhibitors of PG biosynthesis delay castor oil induced diarrhoea. PGE2 causes diarrhoea in experimental animals as well as in human beings [9]. Most recently NO has been claimed to contribute to the inhibitory nor adrenergic, non cholinergic neurotransmitter that mediates gastrointestinal motility in physiological and certain non-pathophysiological states, such as in absorptive and secretory processes. NO could lead to gut secretion via elevation of cGMP and cAMP concentration [13]. AQETB and ETETB might be inhibiting the synthesis of ricinoleic acid as a result of this local irritation and intestinal motility decreased or inhibited and in turn brings the absorption of water, electrolytes and glucose. Apart from this AQETB and ETETB might be also inhibiting the synthesis of PGE2 and NO and/or their activity in turn to prevent diarrhoea even the extracts might produced antidiarrhoeal effect by anti-secretory effect. |
| |
| PGE2 agonist acts on EP1, EP2, EP3, EP4 type of PG receptors [10] and these all prostanoid receptors are G-protein coupled receptor which utilizes the IP3/DAG or cAMP transducer mechanism. EP1 causes smooth muscles contraction through IP3/DAG pathway and EP2 causes smooth muscles relaxation by increasing cAMP [12]. PGE2 causes stimulation of gut motility and conversion of small intestinal mucosa from absorption to secretion of water and electrolytes. PGE2 causes accumulation of fluid in intestinal lumen this fluid is accumulated because of the inhibition of absorption of sodium, chloride and glucose (a major stimulus to intestinal absorption of water and electrolytes) [7, 12, 16]. The AQETB and ETETB might be enhancing the absorption of water, electrolytes and glucose and also brings the antisecretory effect of intestinal mucosa as a result of this antidiarrhoeal effect occured. These extracts might be also inhibiting secretory effect of NO. |
| |
| The gastrointestinal tract is innervated by both the parasympathetic and sympathetic fibers of the autonomic nervous system. The peristalsis movement of gastrointestinal tract is myogenic in character and is mainly initiated by the local reflexes and can occur without neural connections to the brain and spinal cord. The extrinsic nerves to the intestine appear to have a minor role in modulating the peristalsis activity of the organ [17]. As cholinergic stimulation often cause diarrhea by increasing GI motility the significant inhibition of GI motility by the extract LHM suggested its probable mode of action to be the prevention of cholinergic transmission or its anticholinergic effect on gastric mucosa [18]. Both the extracts at the dose of 334 mg/kg have an ability to inhibit the peristaltic movement of GIT results the anti-motility effect which in turn allows the absorption of water and electrolytes as a result of this anti-diarrhoeal effect occur. This effect of the extracts might be due to inhibition of acetylcholine and/or histamine effect on gut. |
| |
| Many plants contain tannins and tannic acid which denature proteins by forming a complex (Protein tannate). This complex coats the intestinal mucosa and makes it more resistant while simultaneously diminishing gastric secretions [19]. Tannins are reported to have various physiological effects like anti-irritant, anti-secretory, anti-phlogistic, antimicrobial, and anti-parasitic [20]. Loperamide is used as the standard drug in our study as it stimulates absorption of fluid, electrolytes, and glucose and reversed PGE2, and cholera toxin-induced secretion to absorption. This opiate analogue had no effect on cholera toxin stimulation of adenylate cyclase activity or the rise of tissue cyclic AMP (cAMP) concentrations [21]. Another standard drug atropine significantly reduces intestinal transit time due to its anticholinergic effect. However, it does not inhibits castor oil induced enteropooling, thereby, suggesting that mediators other than acetylcholine are involved in castor oil-induced enteropooling. Furthermore, a decrease in intestinal transit time with atropine could also be due to reduction in gastric emptying [22]. The anti-diarrhoeal activity of flavonoids has been ascribed to their ability to inhibit intestinal motility and hydro-electrolytic secretion which are known to be altered in this intestinal condition. In vitro and in vivo experiments have shown that flavonoids are able to inhibit the intestinal secretary response, induced by prostaglandins E2. In addition, flavonoids have antioxidant properties which are presumed to be responsible for the inhibitory effects exerted upon several enzymes including those involved in the arachidonic acid metabolism [9]. The spasmolytic effects of flavonoids may have been due to a nonspecific action since they were also found to inhibit acetylcholine and BaCl2, induced contractions. Flavonoids were shown to inhibit electrically-induced contractions (transmural electrical stimulations) and those that are induced by a variety of agonists, such as acetylcholine, 5-HT, histamine and certain prostaglandins [23]. Alkaloids are known to inhibit the release of autocoids and prostaglandin, thereby inhibiting the secretion induced by castor oil. Sesquiterpenes, diterpenes, terpenes and terpenoid derivatives are known to inhibit the release of autocoids and prostaglandis, thereby inhibiting the motility and the secretion induced by castor oil [24]. |
| |
| The result of this investigation revealed that the Terminalia belerica contains pharmacologically active substances with anti-diarrhoeal properties. Thus we presume that AQETB and ETETB can be developed to use for the treatment of diarrhoea. Further studies are required to carry out the identification of the proper constituents that are responsible for the anti-diarrhoeal effect. |
| |
ACKNOWLEDGEMENT
|
| |
| The authors are thankful to Dr. Divakar Goli, Principal, Department of Pharmacy, Achraya & BM Reddy College of Pharmacy, Soldevanhalli, Bangalore, Karnataka, India for providing necessary facilities and cooperation during this research work. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










