Key words
|
| |
| Dioscorea hispida, Dennst. Lipid peroxidation, Ehrlich Ascites Carcinoma (EAC), Anticancer. |
| |
Introduction
|
| |
| The chemotherapy of neoplastic disease as becomes increasingly important in recent years. The relatively high toxicity of most anticancer drugs has fostered the development of supplementary drugs that may alleviate this toxic effect or stimulate the regrowth of depleted normal cells (1). Plants have a long history of use in the treatment of cancer. Plants have played an important role as a source of effective anticancer agent, and it is significant that over 60% of currently used anticancer agents are derived in one way or another from natural sources, including plants, marine organism and microorganisms(2).It was also observed from Ayurvedic literature and ethnobotanical studies that the plant is very useful in treating tumour, prevention of hair from falling and as a diuretics but no scientific investigation has been done in this direction(3-5). Therefore, it was thought worthwhile to carry out preliminary phytochemical screening of DhLE for anticancer activity against Ehrlich Ascites Carcinoma in animal model. |
| |
Material and methods
|
| |
|
Plant source
|
| |
| The leaves of Dioscorea hispida(Dioscoreceae) were collected during the month of May 2010 from Bligirirangana hills of Karnataka, India. The plant material was taxonomically identified and authenticated by Dr. G.Panduranga Murthy, Scientist in charge (Authentification No: CSRF/BRT/007B/2009), Ethno-pharmacological laboratory, Centre for Shridevi Research Foundation, SIET, Tumkur, India. |
| |
|
Preparation of extract
|
| |
| The leaves of Dioscorea hispida were dried under shade and then made in to a coarse powder with a mechanical grinder. The powder was passed through sieve no.40 .The dried powder material (350gm) was defatted with petroleum ether at 60-80°C for 18 hrs to remove low polar compounds. The defatted material was further extracted with 90% ethanol by soxhlet’s extraction for 48hrs at 55ºc . After completion of extraction, the solvent removed by evaporation till the solid mass was obtained. The dried extracts were kept in airtight container and stored in refrigerator (6, 7). |
| |
|
Phytochemical identification Tests
|
| |
| Various chemical tests were performed for the phytochemical identification of the ethanolic extract of DhLE as peer standard procedure (8, 9). |
| |
|
Anticancer activity
|
| |
|
Experimental animals
|
| |
| Swiss albino mice of either sex weighing between 22- 30 g were used. The animals were maintained on the suitable nutritional and environmental condition throughout the experiment. The animals were housed in polypropylene cages with paddy house bedding under standard laboratory condition for an acclimatization periods of 7 days prior to performing the experiment. The animals had access to laboratory chow and water. The experimental protocols were approved by institutional Animal Ethical Committee and a written permission from house ethical committee has been taken to carry out (Reference no. JKKMMRFCP/IAEC//2010/008) and complete this study. |
| |
|
Acute toxicity studies
|
| |
| The procedure was followed by using OECD guidelines 423 (Acute toxic class method) The acute toxic class method is a step wise procedure with 3 animals of single sex per step. Depending on the mortality and / or moribund status of the animals, on average 2-4 steps may be necessary to allow judgment on the acute toxicity of the test animals while allowing for acceptable data based scientific conclusion. |
| |
| The method defined uses doses (5, 50, 300 and 2000 mg / kg body weight) and the results allow a substance to be ranked and classified according to the Globally Harmonized system (GHS) for the classification of chemical which cause acute toxicity. |
| |
|
Procedure
|
| |
| Twelve animals (Swiss albino mice weighing between 22-30 g) were selected for studies. The starting dose level of DhLE was 100 mg/kg body weight per oral. Most of the crude extracts possess LD50 value more than 2000 mg. /kg body weight of animal used. Dose volume of 0.1 ml / 100 gm body weight was administered to the animal orally. After giving the dose, the toxic signs were observed within 3-4 hours. Body weight of animals before and after administration, onset of toxicity and signs of toxicity like changes in skin, fur, eyes, and mucous membrane and also respiratory, circulatory, autonomic, central nervous systems, somatomotor activity and behavior pattern, signs of tremors, convulsion, salivation, diarrhea, lethargy, sleep and coma if any, was observed. |
| |
|
Observation
|
| |
| No toxicity or death was observed for the given dose levels, in selected and treated animals. So the LD50 of the DhLE as per OECD guidelines-423 is greater than 2000mg/kg (LD50 > 2000mg/kg). Hence, the biological dose was fixed at 100 and 200mg/kg body weight for the extract. |
| |
|
Cancer cell line
|
| |
| EAC cells were obtained from Amala Cancer Research Center, Thrissur, Kerala, India. They were maintained by weekly intraperitoneal inoculation of 106 cells per mouse. |
| |
|
Preparation of extract drug and mode of administration
|
| |
| In the present anticancer study, DhLE in the dose of 100mg/kg and 200mg/kg body weight were prepared as suspension by dissolving the DhLE in 1%CMC to get desired concentration (10, 11). |
| |
|
Procedure
|
| |
| Animals were divided into five groups.Group1 containing 6 animals served as the normal control for which inoculation of tumour cells was not done. The remaining animals were inoculated with EAC (2×106) (12) and divided in to 4 groups containing 10 mice in each group. Group second, served as the tumour control. Group third which served as a positive control was treated with 5-Fluro uracil at the dose of 20mg/kg body weight. Group fourth and fifth were treated with DhLE at 100mg/kg and 200mg/kg body weight doses respectively. All the treatments were given orally at 24 hrs after tumour inoculation and continued all treatments on the 11th day, six animals from each group were taken. On 14th day after tumour inoculation the ascitic fluid was collected from the peritoneal cavity and measure the tumour cell packed volume and viable tumor cells counts. Immediately after collecting the ascitic fluid, the mice were killed by cervical dislocation. Then liver was excised, rinsed in ice cold normal saline and used for estimation of lipid peroxidation. The rest of the animals were kept to check survival time and percentage increase in body weight (13, 14, and 15). The details of all the procedures are given below. |
| |
|
Antitumor parameters
|
| |
|
1. Survival time
|
| |
| The effect of DhLE on tumour growth was monitored by recording the mortality daily for a period of 6 weeks and increase in life span was calculated(11,12). |
| |
 |
| |
| Where T= Average number of days the treated animals survived and C=Average number of days control animals survived. |
| |
|
2. The body weight analysis
|
| |
| Body weights of the experimental mice were recorded both in the treated and control groups at the beginning of the experiment (0 day) and sequentially on every 5th day during the treatment period and calculated on 15th day (13). |
| |
 |
| |
|
3. Tumour packed cell volume
|
| |
| The ascitic fluid was collected from the peritoneal cavity. The packed cell volume was measured by taking in a graduated centrifuge tube and by centrifuging at 1000 rpm for 5mins (12). |
| |
|
4. Tumour viable cell count
|
| |
| The ascitic fluid was taken in WBC pipette and diluted 100 times .Then a drop of the diluted cell suspension was placed on the Neubauer counting chamber. The cells were then stained with trypan blue (0.4% in normal saline) dye. The cells that did not take up the dye were viable cells and those that took the stain were nonviable. These viable cells were counted in the 64 small squares (13). |
| |
| After collection of ascitic fluid from the mice, the mice were sacrificed. Then liver was excised, rinsed in ice cold normal saline and used for lipid peroxidation estimation. |
| |
|
5. Measurement of lipid peroxidation (LPO) in liver tissue
|
| |
| The level of Thiobarbituric acid reactive substances (TBARS) in the liver was measured by the method of Ohkawa (16) as a marker for lipid peroxidation. 0.2 ml of liver homogenate was treated with 20% of 1.5 ml of acetic acid (pH 3.5), 1.5 ml TBA and 0.2 ml SDS (8.1%), volume is made up to 5 ml with distilled water. The mixture was then heated at 100 ºC for 60 min. The mixture was cooled and added 5 ml of nbutanol– pyridine mixture (15:1). The mixture was shaken vigorously. After centrifugation of the mixture at 4000 rpm for 10 min, the organic layer was taken and its absorbance was measured at 532 nm. The concentration of MDA formed is expressed as nM/g wet tissue. |
| |
|
6. Statistical Analysis
|
| |
| All the values are expressed as mean ± S.E.M for groups of six animals each. Analyzed by one way ANOVA and compared by using Tukey- Kramer multiple comparison tests. The values are statistically significant at three levels, ***p<0.001. **p<0.01, *p<0.05 But ns if p > 0.05. |
| |
Results
|
| |
|
1. Preliminary phyto-chemical analysis of ethanolic extract of Dioscorea hispida leaves
|
| |
| The result of preliminary phyto-chemical analysis of DhLE revealed that DhLE contain Carbohydrates, proteins, alkaloids, glycosides, saponins, tannins and phenolic compounds. |
| |
|
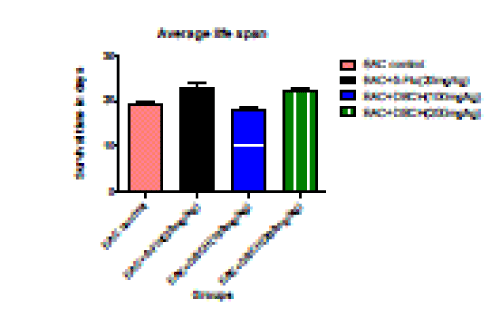
2. Percentage increase in life span
|
| |
| The reliable criteria for judging the value of an anticancer drug is prolongation of the life span of animals. In EAC tumour bearing mice, a regular rapid increase in ascitic tumour volume was observed. In the EAC tumour control group, the average life span of animals was found to be 19.36±0.464 days. DhLE at the dose of 200 mg/kg body weight increases the average life span about 15.26%±2.656 (P<0.001). Standard (5-Flu uracil) found to be 21.152%±5.33 (P<0.001) where as no significant increase was noticed in life span at the dose of 100mg/kg body weight. |
| |
|
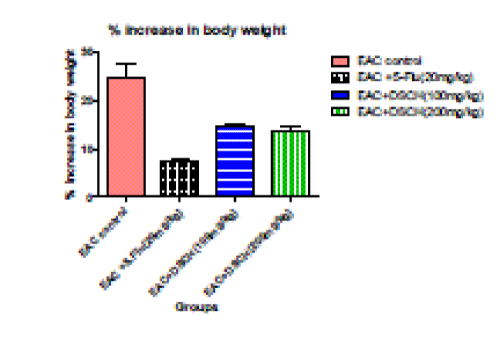
3. Percentage increase in body weight
|
| |
| In the EAC tumour control group percentage increase in body weight is found to be 24.62%±2.75. DhLE at 100mg/kg body weight decreases percentage increase body weight to 14.46%±0.588 (P<0.001).where as in DhLE at 200 mg/ kg body weight decreases percentage increase in body weight to 13.81%±0.625 ( P<0.001). However in Standard found to be 7.43±0.641 ( P<0.001). |
| |
|
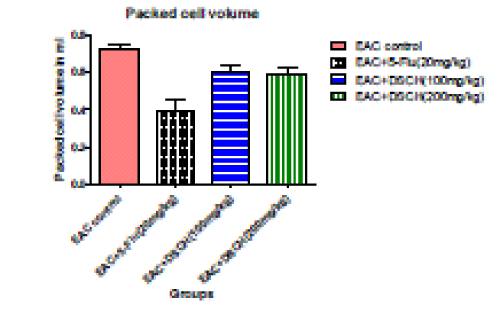
4. Packed cell volume (ml)
|
| |
| In the EAC tumour control group, packed cell volume is found to be 0.722±0.0327. DhLE at 100mg/kg body weight decreases packed cell volume to 0.601±0.03041 (P<0.05).where as in DhLE at 200 mg/ kg body weight decreases packed cell volume Significantly to 0.595±0.0290 ( P<0.001). However, the standard value is found to be 0.394±0.0363 (P<0.001). |
| |
|
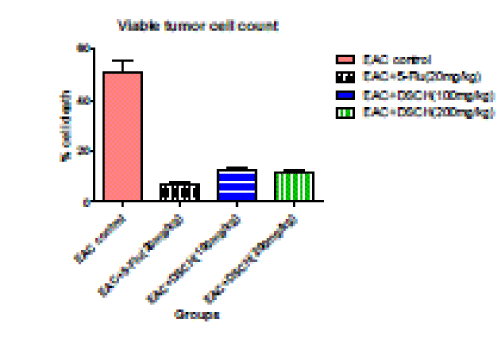
5. Viable tumour cells count
|
| |
| In the EAC tumour control group, viable tumour cell count is found to be 50.83±5.108. DhLE at 100mg/kg body weight decreases viable tumour cell count to 12.16±0.792 (P<0.001).whereas, in DhLE at 200 mg/ kg body weight decreases viable tumour cell count significantly to 11.33±1.085 (P<0.001). However, the standard value is found to be 6.66±0.802 (P<0.001). |
| |
|
6. Lipid Peroxidation
|
| |
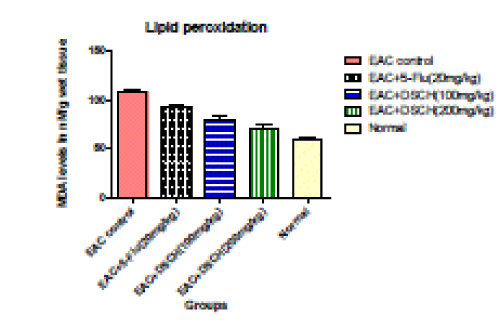
| In the EAC tumour control group, MDA levels nM/g wet tissue is found to be107 .96±2.791. DhLE at 100mg/kg body weight MDA levels nM/g wet tissue decreased to 79.51±3.183 (P<0.001).where as in DhLE at 200 mg/ kg body weight decreased MDA levels nM/g wet tissue Significantly to 70.69±2.965( P<0.001). However, the standard value is found to be 92.86±2.306 (P<0.001). |
| |
Discussions
|
| |
| In the preliminary phyto-chemical screening, the DhLE were found to contain higher amount of saponins and phenolic compounds 2, 17. The presence of these active metabolites represents the plant as an alternative source for therapeutic sectors to formulate a drug1. Further, anticancer potential of the DhLE was estimated in EAC bearing carcinoma cell. DhLE had considerably reduced tumour volume and percentage increase in body weight of the test animals 15, 16. It is also found that, DhLE significantly reduced the elevated levels of lipid peroxidation and thereby it may act as an antioxidant agent 18. |
| |
Conclusion
|
| |
| The DhLE possessed significant anticancer and antioxidant activity due to its higher saponins and phenolic content. Further investigation on different biological activities with different modes will not only validate the types of activities claimed by ayurvedic, siddha and traditional practitioners, but also will bring out innovation in the field of therapeutics. |
| |
Acknowledgement
|
| |
| The authors Sincere thank the Authorities of Center for Shridevi Research Foundation, Tumkur, Karnataka for their guidance, counseling and providing facilities to carry-out Project work. I extend my deep sense of gratitude to Principal and Guide (internal) J.K.K.M.M.R.F College of pharmacy, Komarapalyam for deputing me to carry out the above Project at CSRF, Tumkur-572 106, India. |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |













