Key words
|
| |
| Solanum nigrum, Cardioprotective, Anti-oxidant, fruit extract |
| |
Introduction
|
| |
| Coronary artery disease (CAD) is a leading cause of death in the Western world. Emergence of ndia as a fast developing economy has also resulted in development of “office culture” characterized by sedentary life style which is one of the key implications of CAD. The prevalence of CAD in urban North India varies from 7% to 10% [1] compared to 3% in USA [2] and <1% in Japan [3]. The CAD rates in South India are twofold higher than in North India, with Kerala reporting 14% in urban [4] and 7% in rural Thiruvananthapuram [5]. The treatment of IHD involves expensive and chronic drug therapy or equally expensive interventional procedures such as thrombolytic therapy and surgical recanalization [6], which has its own drawbacks in the form of reperfusion injury [7]. The etiopathogenesis of this phenomenon is complex and multifactorial of which oxygen free radicals (OFR) have been identified as the major contributor [8]. Efforts to contain OFR induced damage, using modern pharmacological agents have met with little success. Awareness of the rising incidence of ischemic heart disease (IHD) in India, coupled with prohibitive cost of treatment, particularly for developing countries has generated urgency for the rapid development of an alternative therapeutic modality which has always been available to us in the form of traditional or folklore medicine. Solanum nigrum Linn. (Solanaceae) is commonly known as ‘Black nightshade’. The plant has been extensively used in traditional medicine in India and other parts of world to cure liver disorders [9], chronic skin ailments (psoriasis and ringworm) [10], inflammatory conditions, menstrual pain, fevers, diarrhea, eye diseases, hydrophobia etc. Keeping in view the need for a systematic scientific evaluation of medicinal plants in the amelioration of IHD, the present study was aimed at evaluating the cardioprotective potential of Solanum nigrum using global in-vitro ischemia-reperfusion injury as the animal model. |
| |
MATERIALS AND METHODS
|
| |
|
Plant materials
|
| |
| The plant Solanum nigrum Linn was collected from Haldia, Purba Medinipur Dt, during the month of March and April. That was identified by Dr. K. Gauthaman M.Pharm, Ph.D, Department of Pharmacognosy, Himalayan Pharmacy Institute, Majhitar, Sikkim, India. The berries were taken and dried in shade for 45 days. Then the shade-dried berries were made into coarse granules and were used for further investigation. |
| |
|
Experimental Animals
|
| |
| Male Albino rats (250-350 gm) were housed in the departmental animal house at an ambient temperature of 25°C, under a 12hour dark -12 hour light, cycle, for the whole period of the study (i.e. twelve weeks) and were fed with standard rat chow from Amrut Laboratory Animal feed, Bangalore and water, ad libitum. Experiments were carried out as per guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India(Reg. No.- 37/ 2007/ CPCSEA). |
| |
|
Chemicals & Reagents
|
| |
| All the chemicals and reagents used for these studies were purchased from Sigma chemicals, USA and SD fine chemicals, India. |
| |
|
Preparation of Extract
|
| |
| Dried fruit’s coarse granules (850 gram) were divided in three parts, treated each three times with fresh methanol (1000 ml) separately for 48 h. The methanolic extracts thus obtained were combined, filtered and distilled on a water bath. The last traces of the solvent were evaporated under reduced pressure in rotatory evaporator (Heidolph Laborota 4011 digital). The yield of the methanolic extract was 6.75 % w/w (58 gram). Pharmacological studies were carried out by suspending a weighed amount of the extract in normal saline (95 ml): tween 80 (5 ml) ratio. |
| |
|
Determination of the test doses
|
| |
| A pilot level study was performed prior to initiation of protocol involving toxicological dose evaluation of alcoholic extract of Solanum nigrum in a dose range starting from 1 mg/Kg p.o. Mild toxic effects were observed at a dose of 7 mg/Kg p.o. Therefore, two median doses viz. 2.5 mg/Kg p.o and 5.0 mg/Kg p.o were selected for the present study. |
| |
|
Determination of cardioprotective activity
|
| |
| The rats were anaesthetized with ether, the chest opened and the heart along with one cm of ascending aorta attached was quickly removed and dipped in ice-cold saline. The hearts were then mounted on Langerdorff's apparatus (AD Instruments Australia) and perfused with Kreb's Hensleitt's (K-H) buffer [11] at a constant pressure of 60-70 mm Hg at 37°C, and aerated with a mixture of O2 (95%) and CO2 (5%). Following an initial period of 5 min of stabilization, the flow was stopped for 9 minutes (ischemia) followed by perfusion with K-H buffer for 12 minutes (Reperfusion) [12, 13]. At the end of ischemiareperfusion, the hearts were cut into two parts and one part was kept at -20°C for TBARS estimation and the other part was kept at -80°C in liquid nitrogen, till they were taken up for the estimation of SOD, reduced glutathione and tissue catalase content. |
| |
|
Estimation of Biochemical Parameters
|
| |
| Hearts from ten rats of each group were harvested and stored in liquid nitrogen for estimations of basal endogenous antioxidants. Roughly 0.5-1.0 gm of tissue was quickly cut out from the previously marked out area after reperfusion and immediately frozen in liquid nitrogen. The whole process was done in 8-10 seconds. The samples for biochemical estimations were weighed in an electronic pan balance and subsequently processed for the estimation of following parameters: |
| |
| Myocardial Thiobarbituric Acid Reactive Substances (TBARS) |
| |
| TBARS levels in the myocardium were determined by a modified version of the method described by Ohkawa et al [14]. Results were expressed as nmol/g wet tissue wt. |
| |
| Myocardial Reduced Glutathione [GSH] Glutathione was estimated by the method described by Ellman et al [15]. Results were expressed as μmole/g wet tissue weight. |
| |
| Estimation of SOD |
| |
| SOD levels in the hearts were determined by the method of McCord and Firdovich [16] and modified by Kakkar et al [17]. Results were expressed as IU/mg protein. |
| |
| Myocardial Catalase |
| |
| Catalase estimation for the tissue sample was done by the method of Aebi, [18]. Results were expressed as IU/mg protein |
| |
| Histopathlogical Study |
| |
| The myocardial tissue of rat from each group was taken and stained with Haematoxylin-Eosin (H-E) stain. After washing, the tissue was mounted on the compound microscope and myofibrillar structures were observed. |
| |
| Statistical Analysis |
| |
| Values are expressed as mean ± SEM; significance is set at P ≤ 0.05. One Way Analysis of variance (ANOVA) was carried out to test the significance of the biochemical data of the different groups in myocardial tissue samples. |
| |
Results
|
| |
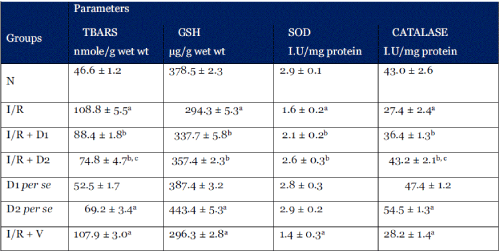
| Table 1: Changes in myocardial tissue Thiobarbituric acid reactive substances (TBARS), Reduced Glutathione (GSH), Superoxide Desmutase (SOD) and Catalase (CAT) of the normal (N), Ischaemia reperfusion (I/R), and Ischaemia Reperfusion followed by Solanum nigrum extract (D1 = 2.5 mg/Kg p.o; D2 = 5.0 mg/Kg p.o), treated and Ischaemia Reperfusion followed by vehicle (I/R + V) treated groups. Results are represented as the mean ± S.E.M. with n = 10 in each group. a p<0.05, as compared to the N group; b p<0.05, as compared to I/R group; c p<0.05, as compared to D1 group |
| |
 |
| |
| Myocardial TBARS |
| |
| Ischemia-Reperfusion injury resulted in significant increase in the levels of myocardial TBARS (108.8 ± 5.5 nmoles/g wet wt; p < 0.001) as compared to the normal group (46.6 ± 1.2 nmoles/g wet tissue wt). Significant increase in the TBARS levels was also seen in the I/R + vehicle group as well as D2 per se group (107.9 ± 3.0 and 69.2 ± 3.4 nmoles/g wet tissue wt respectively). Treatment with methanolic extract of solanum nigrum (2.5 mg/Kg and 5.0 mg/Kg) significantly attenuated Ischemia- Reperfusion injury induced increase in myocardial tissue TBARS levels (88.4 ± 2.8 and 74.8 ± 4.7 nmoles/g wet tissue wt respectively) |
| |
| Myocardial GSH |
| |
| Ischemia-Reperfusion injury resulted in significant decrease in the levels of myocardial GSH (294.3 ± 5.3 μg/g wet tissue wt; p < 0.001 respectively) as compared to normal group (378.5 ± 2.3 mg/g wet wt.). Significant increase in the myocardial GSH levels was also seen in the D2 per se group (443.4 ± 6.4 μg/g wet tissue wt respectively) as compared to normal group (378.5 ± 2.3 mg/g wet wt.). Treatment with methanolic extract of solanum nigrum (2.5 mg/Kg and 5.0 mg/Kg) significantly attenuated Ischemia-Reperfusion injury induced decrease in myocardial tissue GSH levels (337.7 ± 5.8 and 357.5 ± 2.4 μg/g wet tissue wt respectively). |
| |
| Myocardial Superoxide dismutase (SOD) |
| |
| Ischemia-Reperfusion injury resulted in significant decrease in the levels of myocardial superoxide dismutase (1.6 ± 0.2 I.U/mg protein) as compared to normal group (2.9 ± 0.1 I.U/mg proteins). Treatment with methanolic extract of solanum nigrum (2.5 mg/Kg and 5.0 mg/Kg) significantly attenuated Ischemia-Reperfusion injury induced decrease in myocardial tissue SOD levels (2.1 ± 0.2 and 2.6 ± 0.3 I.U/mg protein respectively) |
| |
| Myocardial Catalase |
| |
| Ischemia-Reperfusion injury resulted in significant decrease in the levels of myocardial catalase (27.4 ± 2.4 I.U/mg protein) as compared to normal group (43.0 ± 2.6 I.U/mg protein). Treatment with methanolic extract of solanum nigrum (2.5 mg/Kg and 5.0 mg/Kg) significantly attenuated Ischemia- Reperfusion injury induced decrease in myocardial tissue catalase levels (36.4 ±1.3 and 43.2 ± 2.1 I.U/mg protein respectively). Significant increase in the myocardial catalase levels was also seen in the D2 per se group (54.5 ± 2.1 I.U/mg protein) as compared to normal group (43.0 ± 2.6 I.U/mg protein). |
| |
 |
| |
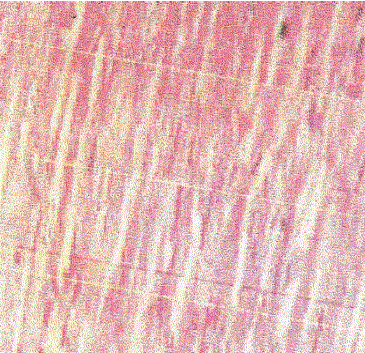
| Slide 1: Normal rat myocardium showing well maintained myofibrillar structures. |
| |
 |
| |
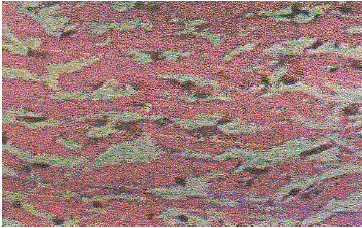
| Slide 2: Myocardium of the Rat subjected to I-R injury showing extensive degeneration of myofibrils with leukocytic accumulation, Edema and vacuolization. |
| |
 |
| |
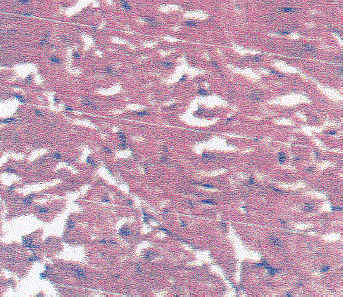
| Slide 3: Myocardium of the rat treated with low dose (2.5 mg/Kg) methanolic extract of Solanum nigrum perse. |
| |
 |
| |
| Slide 4: Myocardium of the rat treated with high dose (5.0 mg/Kg) methanolic extract of Solanum nigrum per se. |
| |
 |
| |
| Slide 5: Myocardium of the rat subjected to I-R injury and treated with low dose (2.5 mg/Kg) of methanolic extract of Solanum nigrum. |
| |
| Slide 6: Myocardium of the rat subjected to I-R injury and treated with high dose (5.0 mg/Kg) of methanolic extract of Solanum nigrum. |
| |
Discussion
|
| |
| In the present study, Ischemia-Reperfusion injury resulted in an increase in the oxidative stress as evidenced by increase in myocardial TBARS and depletion of myocardial endogenous antioxidants enzymes (GSH, SOD and CAT). Chronic administration of the methanolic extract of Solanum nigrum at both the employed doses protected the hearts from ischemia-reperfusion induced oxidative stress, as evidenced by conservation of endogenous antioxidant enzyme levels and prevention in rise of TBARS to a significant extent. Ischemic-reperfusion injury is commonly associated with various stages of ischemic heart disease, starting from stable angina to acute myocardial infarction with spontaneous or induced reperfusion [19] with oxidative stress playing a central role in its etiopathology of this condition [20]. This suggests that chronic administration of the extract imparted better oxidant stress bearing capacity in the rat hearts, by enhancing endogenous antioxidant. |
| |
| Chronic per se oral administration of alcoholic extract of Solanum nigrum (at a dose of 5.0 mg/Kg) caused an increase in basal GSH and CAT levels which indicated its possible adaptogenic property. However, one unique finding of the present study is that per se administration of alcoholic extract of Solanum nigrum was associated with a concominant rise in the TBARS levels. Although rise in TBARS is indicative of increased oxidative stress, it did not cause any overt cellular injury, as evidenced by histopathological study. It is, therefore possible that the increase in TBARS level was non-lethal and might be responsible for cellular adaptive mechanisms, leading to increased synthesis of endogenous antioxidants. Because, previously it has been reported that low levels of oxidative stress, cytokines, endo-toxins stimulate the synthesis of cellular antioxidants [21, 22, 23]. The major chemical compounds, identified in the alcoholic extract of Solanum nigrum were flavonids and glycosides and have significant antioxidants properties [24, 25]. However, many investigators are of the opinion that any antioxidant compound can also behave as a pro-oxidant [26, 27]. In this regard, melatonin has been shown to exert both pro- and antioxidant effects depending on the concentration and duration of exposure [28]. Augmentation of basal endogenous antioxidant reserve by any therapeutic agent is presently the focus of great scientific interest [29, 30] as it is expected to offer better protection against oxidative stress than any exogenously administered antioxidants. It has also been shown that transgenic mice overexpressing CuZn-SOD are more resistant to cardiac injury, than mice, which are exogenously administered with SOD [31]. Moreover, increase in GSH and catalase, as observed in the present study, is supposed to be more beneficial than SOD, because increase in SOD without a concomitant increase in catalase may lead to intracellular accumulation of H2O2 [32]. Among medicinal plants, similar effects have been observed with chronic administration of garlic [33]. In the present study the isolated perfused rat heart as a model was used to investigate the cardioprotective action of Solanum nigrum, and the choice of the preparation is very wide and best characterized. This model was also the most frequently used one for the development and assessment of anti ischemic agents. |
| |
| Chronic oral administration of alcoholic extract of Solanum nigrum in rat augments myocardial endogenous antioxidants, without causing any cellular injury. It has a definite, dose-dependent modulatory effect on the antioxidant milieu of heart. The drug treated groups offered better cardioprotection when subjected to IR injury. There was also no definite pattern of change in the level of TBARS and the endogenous antioxidants. Hence these modulatory actions seem to be enzymespecific. The study reveals an important cardioprotective action of the Solanum nigrum, a widely used Indian medicinal plant in IHD. |
| |
| The present study reveals important cardioprotective of action of the alcoholic extract of Solanum nigrum, a widely used Indian medicinal plant in ischemic heart disease. |
| |
Acknowledgement
|
| |
| The authors would like to heartily thank Er. S.K Punj, Chancellor, Sri Sai University, Palampur and Mrs. Tripta Punj, M.D, Sri Sai Group of Institutions, Badhani, Pathankot for their unending support and inspiration towards this research work. |
| |












