Key words
Chronic obstructive pulmonary disease; diaphragm; transcranial magnetic stimulation; phrenic nerve conduction; corticospinal pathways.
Abbreviations
COPD: chronic obstructive pulmonary disease; TMS: transcranial magnetic stimulation; MEP: motor evoked potential; CMCT: central motor conduction time; CMS: cervical magnetic stimulation; PMEPL: peripheral motor evoked potential latency; CMEPL: cortical motor evoked potential latency; GOLD: Global Initiative for Chronic Obstructive Lung Disease; DRMT: diaphragmatic resting motor threshold; BMI: body mass index; FVC%: predicted forced vital capacity; FEV1%: predicted forced expiratory volume in the 1st second; PaCO2: partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen; HCO-3: bicarbonate; Na+: sodium; K+: potassium; Cl-: chloride.
Introduction
Chronic obstructive pulmonary disease (COPD) is the sixth cause of death in the world and affects 4-6% of people more than 45 years of age. Ventilatory failure remains the most common cause of death with COPD. COPD is a chronic medical illness characterized by partial reversible obstruction of large and small airways and effort-independent limitation of expiratory airways with forced exhalations (that do not change markedly over periods of several months of observation), frequent attacks of exacerbation and hospital admissions ended by respiratory failure. Fixed airway limitation in COPD is caused by destruction of alveolar walls and peribronchiolar fibrosis with reduced lung elastic recoil [1]. Diaphragm is the main inspiratory muscle agonist. There are several evidences that the diaphragm and other respiratory muscles are able to express adaptive changes early in the disease course in response to pulmonary hyperinflation [2]. However, imbalance between respiratory muscle overload and adaptation may occur in response to persistent hyperinflation, increase of disease severity, increments of mechanical or metabolic loads, poor energy supply and recurrent infection and exacerbations [3]. The imbalance between inspiratory muscle load and capacity, places the muscles at mechanical disadvantage resulting in diaphragmatic fatigue or weakness [4], shortness of breath, exertion intolerance and hypoventilation [5]. This myogenic phenomenon was commonly accepted as a mechanism of hypercapnic respiratory failure in COPD [6]. However, the discrepancy between in vivo and in vitro results makes this myogenic assumption with COPD less likely as a sole mechanism and the exact pathophysiology of diaphragm dysfunction is still unclear [7].
It has been suggested that the contractile function of diaphragm fibers may be impaired in early stages of COPD [8]. This is supported by the following findings: a) many patients develop respiratory failure and require hospital admission even if the cause of the exacerbation is less dramatic as in the presence of bronchial infection, pain of any nature, etc., which means that loss of balance between respiratory muscle overload and adaptation is not always associated with extreme situations [7-9]. This can be explained by the fact that, the diaphragm contractile performance in vivo is also determined by many factors which include: the corticospinal neural drive, phrenic nerve function, neuromuscular transmission, contractile function of a single fiber, muscle fiber recruitment, calcium homeostasis and others [8, 10-12].
Little is known about the efficacy of the neural drive to the diaphragm and its possible involvement in diaphragmatic decompensation in patients with COPD [10-12,13]. Throughout the past decade, magnetic stimulation has proved to be technically easier to apply and can be performed repeatedly in elderly patients with respiratory disease [8-12]. Diaphragmatic contractile function had been previously assessed by magnetic stimulation of the phrenic nerve roots in the neck in COPD patients [8,14-17]. Transcranial magnetic stimulation (TMS) evokes a motor evoked potential (MEP) that expresses corticospinal pathway function [18].
Finding the triggers for diaphragmatic dysfunction in COPD is especially challenging because several demographic, clinical and laboratory variables (as age, duration of illness, smoking habits, degree of hypoxemia and electrolyte disturbance) and structural and functional diaphragmatic abnormalities may be present early in the course of the disease. This information may help in improving the quality of life and decrease morbidity and mortality among patients with COPD.
Aim of the study
Due to dearth of evidence, we systematically assessed the cortical response of the diaphragm (an indicator of its neural pathway function) in patients with mild and moderate and stable COPD using magnetic stimulation.
Materials and methods
This case-control study included 20 male patients (mean age: 52.80 ± 3.30 years) with mild-to-moderate COPD [Global Initiative for Chronic Obstructive Lung Disease (GOLD) grade I/II] [19]. COPD was diagnosed on the basis of clinical history, physical examination, pulmonary function tests, chest radiograph and chest computed tomography. Patients were recruited from the out-patients clinic of Internal Medicine department of Assiut University Hospital, Assiut, Egypt., during their follow up visits. Twenty age-matched (mean age: 54.40±3.35 years) healthy male subjects randomly selected from the general population were included as controls for comparison. Patients were on their usual medications including inhaled bronchodilators and were clinically stable for at least 3 months prior to the time of study. Excluded were patients with: 1) bronchial asthma, bronchiectasis, and interstitial lung diseases, 2) other medical illness (as diabetes mellitus, uremia, hepatic failure, etc), 3) neurological disease (as a complication of cerebrovascular stroke, dementia, epilepsy, peripheral neuropathy, muscle disease, etc), 4) psychiatric illness, 5) morbid obesity, 6) drug abuse, 7) neoplasm, 8) neurosurgical operation, 9) chronic medications other than that used for COPD that might have effect on muscle function and structure (as systemic corticosteroids, calcium channel blockers, etc) and 10) presence of contraindications for magnetic stimulation (as skull defects, metal in head or neck, electronic devices as pace makers, etc).
The study protocol was approved by the local ethics Committee of Assiut and Al-Azhar faculties of medicine, Assiut, Egypt and informed consent was given by all participants.
Methods
Patients and control subjects underwent: 1) detailed medical and neurological histories and examinations. The respiratory history included questions related to smoking habit, productive cough, breathlessness and use of bronchodilators. 2) chest X- ray, 3) pulmonary function tests were performed with conventional spirography using Sensor Medics Corporation Spirometer (Model CA92687, SN 54065, Osaka, Japan). The predicted forced vital capacity (FVC%), predicted forced expiratory volume in the 1st second (FEV1%) and FEV1/FVC% ratio, were measured [20], 4) arterialized capillary blood gases tension was determined using instrumental laboratory equipment (ABL 600 Radiometer, Copenhagen Denmark) as follow: arterial blood sample was taken from the radial artery to measure pH, partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2) and serum bicarbonate (HCO3). Absolute values of PaO2 >75 mmHg and PaCO2 ranged from 40 to 42 mmHg, were considered normal [21], 5) serum sodium (Na+), potassium (K+) and chloride (Cl-) levels were measured, 6) brain computed tomography (CT) or magnetic resonance imaging (MRI) scanning were done whenever indicated, and 7) electromyography (EMG) was done using needle electrode to lower limb muscles (quadriceps, gluteus maximus and extensor digitorum brevis) to determine whether there was evidence of myopathy or not. Nerve conduction velocity study (NCVS) (motor and sensory) and F-waves were done in both upper and lower limbs as described before [22] to determine whether there was evidence of peripheral neuropathy or not.
Assessment of diaphragmatic neural function
Transcranial magnetic stimulation (TMS) of the diaphragm motor cortex area and cervical magnetic stimulation (CMS) of the phrenic nerve roots in the neck were performed bilaterally. Cortical motor evoked (MEP) response of the diaphragm was recorded contralateral to the stimulated hemisphere.
Device
A commercially available stimulator, Dantec Maglite TM with a flat figure of eight coil (the outer diameter of each wing is 9 cm; peak magnetic field strength is 1.5 Tesla) was used for magnetic stimulation (Dantec Medical, Skovelund, Denmark). The current in the central axis (0) had twice the magnitude of the current flowing in the two arms of the coil [23]. The recording equipment was utilized with a sensitivity of 20μV/ division and filters of 2-2000 Hz.
Procedures
Subjects were seated comfortably in an armchair. For magnetic stimulation of the motor cortex, the coil was applied to the scalp region and a flexible thin plastic grid with coordinates marked 1 cm apart from each other was attached to the scalp, for stimulation of the areas corresponding to the diaphragm. The coil was held tangentially to the skull. CZ point of the International 10-20 electroencephalography (EEG) system is located at the vertex. The stimulus intensity was adjusted to obtain the largest reproducible responses (range: 65-100% stimulator output). Ten stimuli were delivered at each site at a frequency of about 0.3 Hz. The angle of the coil around an optimal site was changed until the highest diaphragmatic compound motor potential was recorded. After establishing the threshold intensity, sites adjacent to this point were stimulated in order to exclude the possibility that other consistent or larger responses could be obtained. If any larger responses occurred, the threshold was again determined at that point and the procedure was repeated. The largest response was obtained at a stimulus coil orientation of 0-90 degrees. The average point of optimal excitability was determined to be 3 cm lateral to mid-line and 2-3 cm anterior to auricular plane [24]. Magnetic trans-cervical stimulation was delivered while the center of the figure of eight was 2 cm lateral to mid-line 1-2 cm above 5th cervical spine with the handle pointing towards the feet and the patient head slightly bent forward to stimulate the phrenic nerve.
Recording
MEPs were recorded from the contralatral diaphragm during the TMS using active surface electrodes placed in the 7th and 8th right and left intercostal spaces, respectively, approximately on the anterior axillary line, with the reference electrode positioned on the corresponding lower rib. A ground electrode was placed on the lower portion of the manubrium sterni [25]. The following parameters were measured: a) Diaphragmatic resting motor threshold (DRMT) reflects the intensity of lowest stimulus needed to activate the most excitable corticospinal elements and evokes a clear MEP of 100 μV in 50% of 10–20 consecutive stimuli when the muscle is at rest. It is expressed as a percentage of the magnetic stimulator maximal output (equal 100%), b) cortical motor evoked potential latency (CMEPL) (millisecond or ms) was measured at the onset of first negative deflection of the evoked response, C) CMEP amplitude (CMEPA) was measured peak-to-peak. CMEPs variables were recorded at deep inspiration (facilitation), d) peripheral motor evoked potential latency (PMEPL) and PMEP amplitude (PMEPA) were also recorded from both sides, and e) central motor conduction time (CMCT) was calculated as follow: (CMCT = CMEPL – PMEPL) [18].
We studied hand muscles in the milder grades of COPD as described before [26] to evaluate whether the neural function of the diaphragm plays a role in the pathophysiology of exacerbation and whether there was subclinical muscle or neuronal involvement before the occurrence of ventilatory insufficiency.
Statistical analysis
Statistical Science for Social Package (SPSS version 12, SPSS Inc., Chicago, IL) was used for data analysis. Data were presented as mean (SD), or number (%). For comparison of two groups, the parametric student “t” test for independent variables was used. Pearson’s correlation coefficient was used to correlate parametric variables. For all tests, a probability P<0.05 was considered significant.
Results
Table (1) showed demographic, spirometric, gasometric and electrolytes findings of the studied groups. It showed that half of the patients (n = 10) had mild COPD (stage I) (FEV1%: 60-79%, and FEV1%/FVC%: <0.7) and the other half (n = 10) had moderate COPD (stage II) (FEV1%: 40–59%; FEV1%/ FVC%: <0.7). Patients had normal EMGs, NCVSs and brain neuroimaging (CT or MRI). Although, we found abnormalities in TMS results of the hand muscles, however, they did not reach statistically significant levels (data not shown). Table (2) showed the results of CMEP and PMEP variables of the studied groups. It showed that compared to control subjects, patients had significant bilateral increase in CMEPL and CMCT (P<0.0001), while DRMT was significantly decreased (P<0.0001). while no differences were identified in CMEPA, PMEPL and PMEPA. Among patients, 45% (n = 9) had delayed CMEPL, 30% (n = 6) had reduced CMEPA, 15% (n = 3) had delayed PMEPL, 25% (n = 5) had reduced PMEPA. For comparison between patients and controls in CMCT and DRMT, only 12 patients were included. As we excluded patients with delayed PMEPL (n = 3) and reduced PMEPA (n = 5). It is possible that slow conduction of the intra-canalicular part of the cervical roots might result in prolonged PMEPL and thus prolonged CMCT. Also for the same reason, patients with reduced PMEPA are not suitable to calculate DRMT. Table (3) showed the correlations between clinical, functional, gasometric values and CMEPs variables in patients with COPD (n = 12). It showed significant correlations between: 1) CMEPL and FEV1% (r = -0.368, P<0.020) and FEV1%/FVC% (r = -0.463, P <0.003), 2) CMEPA and FVC% (r = 0.326, P<0.038), FEV1% (r = 0.563, P<0.0001), FEV1%/FVC% (r = 0.710, P<0.0001) and CMEPL (r = -0.417, P<0.007), and 3) CMCT and FVC% (r = -0.316, P<0.047), FEV1% (r = -0.397, P<0.011), FEV1%/FVC% (r = -0.395, P <0.012), CMEPL (r = 0.741, P<0.0001) and DRMT (r = -0.337, P<0.034).
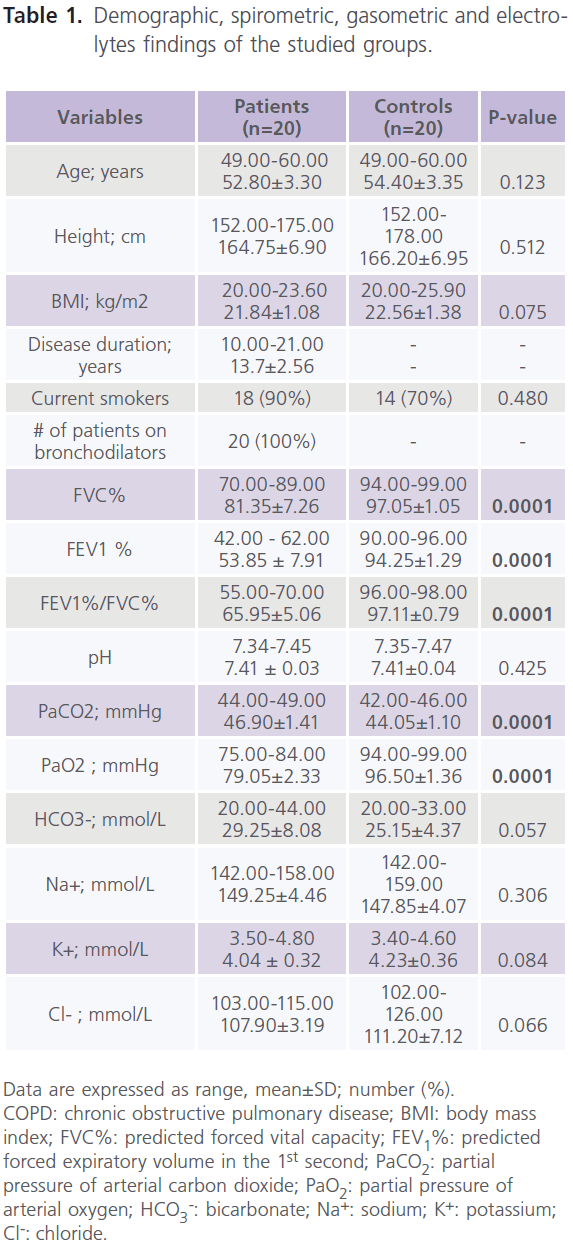
Table 1: Demographic, spirometric, gasometric and electrolytes findings of the studied groups.
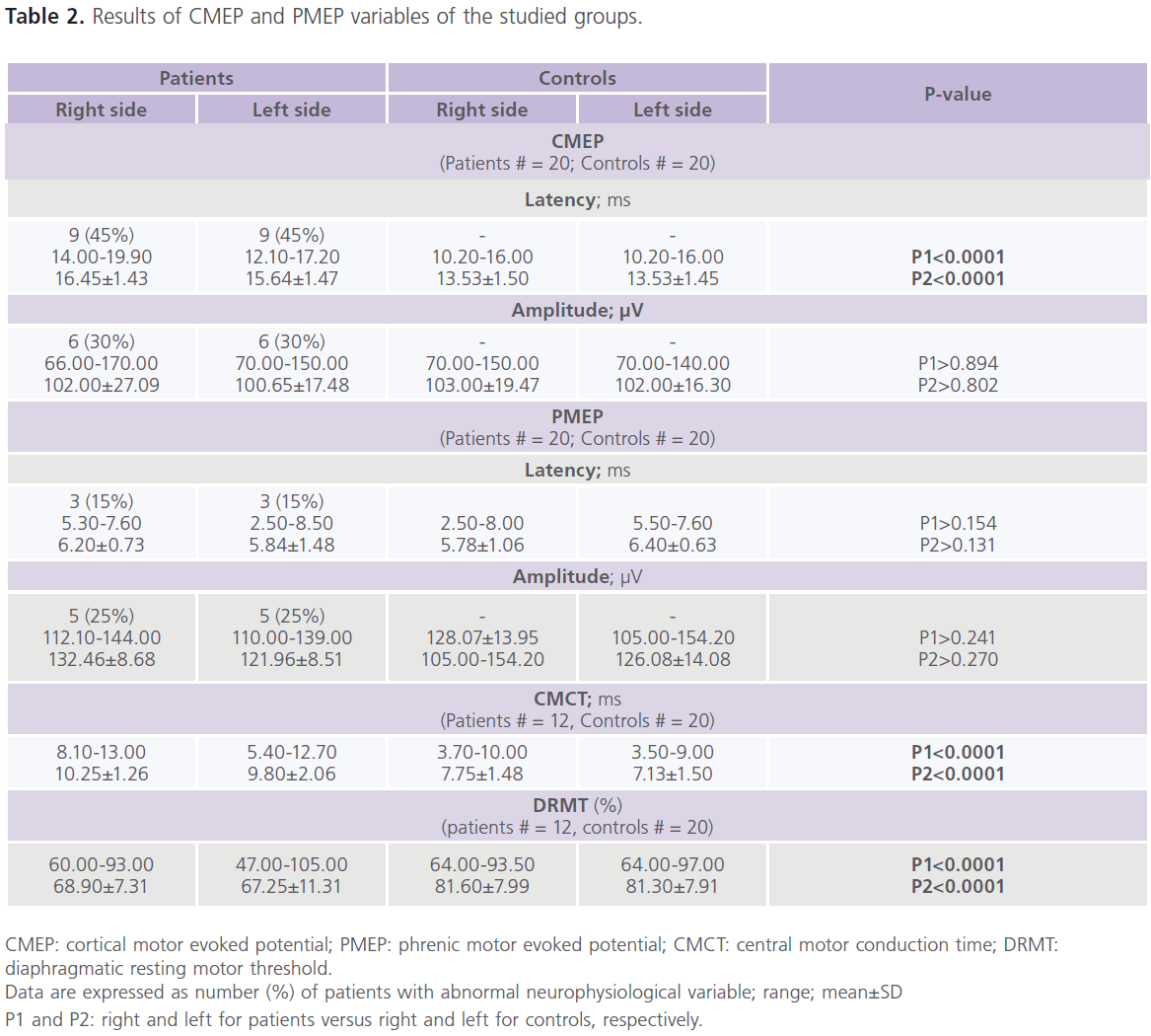
Table 2: Results of CMEP and PMEP variables of the studied groups.
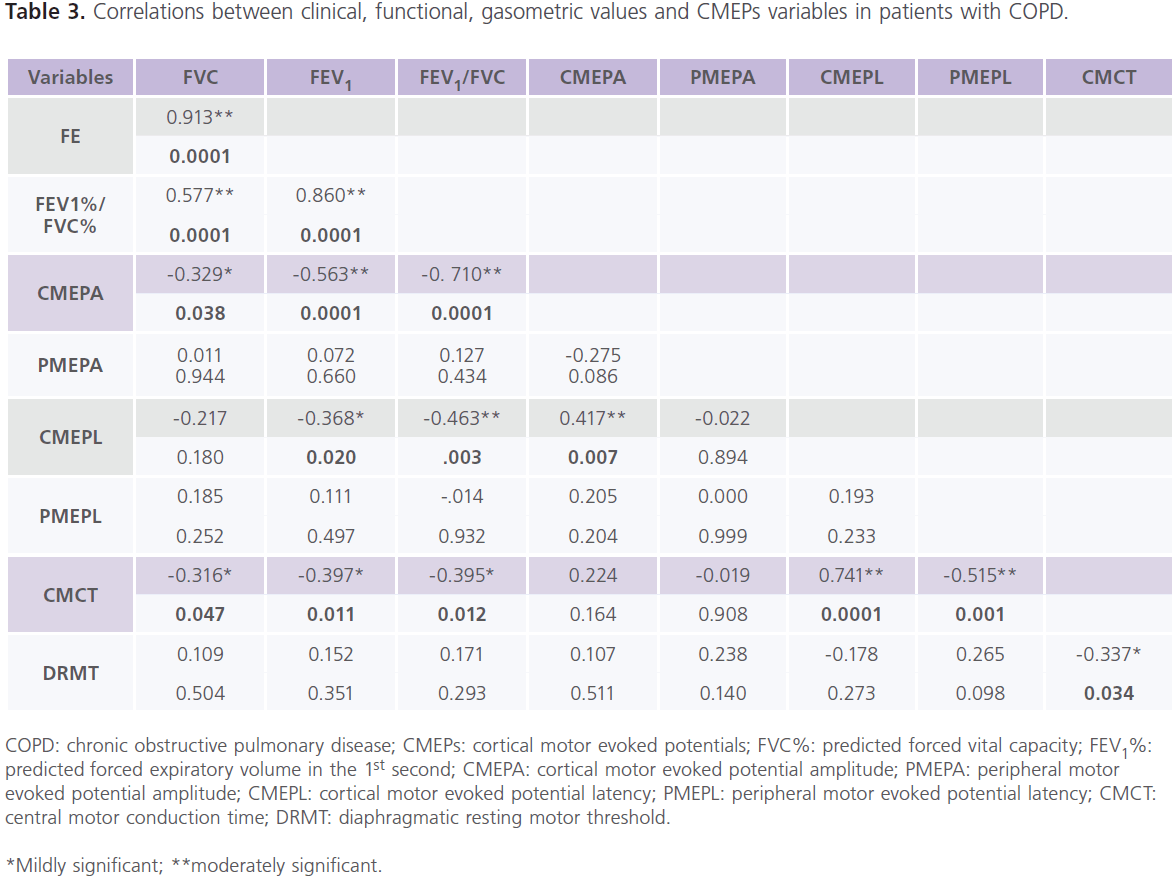
Table 3: Correlations between clinical, functional, gasometric values and CMEPs variables in patients with COPD.
Data are expressed as range, mean±SD; number (%). COPD: chronic obstructive pulmonary disease; BMI: body mass index; FVC%: predicted forced vital capacity; FEV1%: predicted forced expiratory volume in the 1st second; PaCO2: partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen; HCO3 -: bicarbonate; Na+: sodium; K+: potassium; Cl-: chloride.
Discussion
The results of this study indicate functional impairment of the central corticospinl pathways to the diaphragm in patients with mild/moderate COPD as evidenced by the followings: First: hyperexcitability of the diaphragm motor area: which was evidenced by reduced DRMT and inverse correlation between CMCT and DRMT. It has been suggested that chronic increase in respiratory load is a result of hyperinflation. The latter reduces the flow and pressure-generating capacity of the diaphragm. This is compensated by increase in neural drive (leading to increase in firing rates and recruitment), adaptations of the chest wall and diaphragm shape to accommodate the increase in volume, and adaptations of muscle fibers to preserve strength and increase endurance particularly during periods of exacerbation and hypoxia [12]. These limit the impact of hyperinflation on the diaphragm’s ability to generate flow and volume changes [27]. This is also consistent with the followings: a) in animal models of inspiratory resistive loading to apnea, central fatigue [which occurred prior to significant peripheral diaphragm fatigue] was found to play an important role in the development of ventilatory failure [28], b) using twitch interpolation intervention, some authors reported presence of normal or increased levels of diaphragm voluntary activation with stable COPD [29]. The presence of ‘a ceiling’ may render patients with COPD more vulnerable to increase in load during acute exacerbations
[30], c) In humans, it was shown that nearly 50% of force reduction [which occurs during a fatiguing inspiratory task] is due to failure of voluntary motor and central drives [31] as assessed by twitch interpolation techniques after failure of weaning from mechanical ventilation [32], and d) It was observed that when work of breathing is reduced or abolished in healthy subjects, there was decrease in excitability and increase in intra-cortical facilitation which is opposite to what was observed with COPD [33]. Second: we reported bilateral prolongation in CMEPL and CMCT in 45% and reduction in CMEPA in 30% with COPD [10] which were also correlated to the severity of airflow limitation. Third: we and others showed that hypoxemia and hypercarbia [in which smoking is a main risk factor] were associated with impairment of corticospinal pathway and brain excitability in COPD [12-26, 34).
Despite the strengths observed in our study, the relevance of data is limited by the followings: a) the small sample size. This is explained by the long list of inclusion and exclusion criteria, b) caution should be considered in generalizing that dysfunction of diaphragmatic neural drive occur early in COPD, because important variables have to be considered including age, smoking habits, number of exacerbations and presence or absence of respiratory failure, c) CMEPL was delayed about 2-2.5 ms in respect to the control population. Within this range of abnormalities, CMCT prolongation could be due to a delay in the corticospinal tract or by a reduced possibility to recruit I waves, and d) DRMT was higher than controls and it was abnormal in 55% (n = 11) of COPD patients. A possible interpretation is the failure to properly recruit I waves during TMS due to higher threshold.
Conclusions
The results of this study indicate that central impairment (corticospinal dysfunction) occurs early in stable COPD. This is important for predicting disease outcome and emphasizes the importance of early therapeutic interventions before the occurrence of manifest ventilatory insufficiency.
Conflicts of interest
None
2067
References
- Ai-Ping, C., Lee, KH., Lim, TK. In-hospital and 5-year mortality of patients treated in the ICU for acute exacerbation of COPD: a retrospective study. Chest 2005; 128 (2): 518-524.
- Ottenheijm, CA., Heunks, LM., Dekhuijzen, RP. Diaphragm adaptations in patients with COPD. Respir Res 2008; 9: 12.
- Wijnhoven, JH., Janssen, AJ., van Kuppevelt, TH., Rodenburg, RJ., Dekhuijzen, PN. Metabolic capacity of the diaphragm in patients with COPD. Respir Med 2006; 100 (6): 1064-1071.
- Scano, G., Grazzini, M., Stendardi, L., Gigliotti, F. Respiratory muscle energetics during exercise in healthy subjects and patients with COPD. Respir Med 2006; 100 (11): 1896-1906.
- Stubbings, AK., Moore, AJ., Dusmet, M., Goldstraw, P., West, TG., Polkey, MI., Ferenczi, MA. Physiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary disease. J Physiol 2008; 586 (10): 2637-2650.
- Hershenson, MB., Kikuchi, Y., Tzelepis, GE., McCool, FD. Preferential fatigue of the rib cage muscles during inspiratory resistive loaded ventilation. J Appl Physiol 1989; 66 (2): 750-754.
- Levine, S., Gregory, C., Nguyen, T., Shrager, J., Kaiser, L., Rubinstein, N., Dudley, G. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol 2002; 92 (3): 1205-1213.
- Martínez-Llorens, J., Coronell, C., Ramírez-Sarmiento, A., Orozco- Levi, M., Espadaler, JM., Bautista Gáldiz, J., Gea, J. ENIGMA in COPD. Determination of maximal diaphragm strength in chronic obstructive pulmonary disease: cervical magnetic stimulation versus traditional sniff maneuver. Arch Bronchoneumol 2006; 42 (10): 509-515.
- Orozco-Levi, M. Structure and function of the respiratory muscles in patients with COPD: impairment or adaptation? Eur Respir J. 2003; 46 (Suppl.): 41s-51s.
- Lu, Z., Tang, X., Huang, X. Phrenic nerve conduction and diaphragmatic motor evoked potentials: evaluation of respiratory dysfunction. Chin Med J. 1998; 111 (6): 496-499.
- Kayacan, O., Beder, S., Deda, G., Karnak, D. Neurophysiological changes in COPD patients with chronic respiratory insufficiency. Acta Neurol Belg 2002; 101 (3): 160-165.
- Hopkinson, NS., Sharshar, T., Ross, ET., Nickol, AH., Dayer, MJ., Porcher, R., Jonville, S., Moxham, J., Polkey, MI. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respiratory Physiology and Neurobiology 2004; 411 (1): 1-12.
- Mills, GH., Kyroussis, D., Hamnegard, CH., Wragg, S., Polkey, MI., Moxham, J., Green, M. Cervical magnetic stimulation of the phrenic nerves in bilateral diaphragm paralysis. Am J Respir Crit Care Med 1997; 155 (3): 1565-1569.
- 14-. Ferguson, GT. Use of twitch pressures to assess diaphragmatic function and central drive. J Appl Physiol 1994; 77 (4): 1705-1715.
- Hamnegard, CH., Wragg, SD., Mills, GH., Kyroussis, D., Polkey, MI., Bake, B., Moxham, J., Green, M. Clinical assessment of diaphragm strength by cervical magnetic stimulation of the phrenic nerves. Thorax 1996; 51 (12): 1239-1242.
- Laghi, F., Harrison, M., Tobin, MJ. Comparison of magnetic and electrical phrenic nerve stimulation in assessment of diaphragmatic contractility. J Appl Physiol 1996; 80 (5): 1731-1742.
- Similowski, T., Duguet, A., Straus, C., Attali, V., Boisteanu, D., Derenne, JP. Assessment of the voluntary activation of the diaphragm using cervical and cortical magnetic stimulation. Eur Respir J. 1996; 9 (6): 1224-1231.
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000; 406 (6792): 147-150.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease NHLBI/WHO Workshop report. Revised edition. 2006. www.goldcopd.com. [Date last accessed November 25 2006].
- Pauwels, RA., Buist, AS., Calverley, PM., Jenkins, CR., Hurd, SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Nhlbi/who global initiative for chronic obstructive lung disease (gold) workshop summary. Am J Respir Crit Care Med 2001; 163 (5): 1256-1276.
- Cerveri, I., Zoia, MC., Fanfulla, F., Spagnletti, L., Berrayah, L., Grassi, M., Tinelli, C. Reference values arterial oxygen tension in the middleaged and elderly. Am J Respir Crit Care Med 1995; 152 (3): 934-941.
- Wilbourn, AJ., Ferrante, MA. Clinical electromyography. Joynt, RJ., Griggs, RC. (eds.). Clinical Neurology, vol. 1 (looseleaf) Philadelphia, Lippincott-Raven. 1997. pp. 1-78.
- Rösler, KM., Hess, CW., Heckmann, R., Ludin, HP. Significance of the shape and size of stimulating coil in magnetic stimulation of the human cortex. Neuroscience Lett 1989; 100 (1-3): 347-352.
- Maskill, D., Murphy, K., Mier, A., Owen, M., Guz, A. Motor cortical representation of the diaphragm in man. J Physiol 1991; 443: 105-121.
- Demoule, A., Verin, E., Locher, C., Derenne, JP., Similowski, T. Validation of surface recordings of the diaphragm response to transcranial magnetic stimulation in humans. J Appl Physiol 2003; 94 (2): 453-461.
- Mohamed-Hussein, AA., Hamed, SA., Abdel-Hakim, N. Central Cortical Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Transcranial Magnetic Stimulation. Int J Tuberc Lung Dis 2009; 11 (5): 515-521.
- McKenzie, DK., Butler, JE., Gandevia, SC. Respiratory muscle function and activation in chronic obstructive pulmonary disease. J Appl Physiol 2009; 107 (2): 621-629.
- Sassoon, CS., Gruer, SE., Sieck, GC. Temporal relationships of ventilatory failure, pump failure, and diaphragm fatigue. J Appl Physiol 1996; 81 (1): 238-245.
- Topeli, A., Laghi, F., Tobin, MJ. The voluntary drive to breathe is not decreased in hypercapnic patients with severe COPD. Eur Respir J 2001; 18 (1): 53-60.
- Bellemare, F., Bigland-Ritchie, B. Central components of diaphragmatic fatigue assessed by phrenic nerve stimulation. J Appl Physiol 1987; 62 (1): 1307-1316.
- Laghi, F., Cattapan, SE., Jubran, A., Parthasarathy, S., Warshawsky, P., Choi, Y-SA., Tobin, MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 2003; 167 (2): 120-127.
- Sharshar, T., Ross, E., Hopkinson, NS., Porcher, R., Nickol, AH., Jonville, S., Dayer, MJ., Hart, N., Moxham, J., Lofaso, F., Polkey, MS. Depression of diaphragm motor cortex excitability during mechanical ventilation. J Appl Physiol 2004; 97 (1): 3-10.
- Gandevia, SC., Rothwell, JC. Activation of the human diaphragm from the motor cortex. J Physiol 1987; 384: 109-118.
- Kim, HC., Mofarrahi, M., Hussain, SNA. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. International Journal of COPD 2008; 3 (4): 637-658.








