Keywords
Point-of-care testing POCT; i-CHROMA™; CReactive protein; Urine microalbumin
Introduction
The current NHS treatment pathway for the management of a suspected respiratory tract infection provides the decision to prescribe antibiotics based on medical history, clinical examination and assessment of risk [1]. The NICE guidelines on the diagnosis and management of pneumonia in adults recommends that point of care C-Reactive Protein (CRP) testing should be considered for people with symptoms of lower respiratory tract infection in calmly care if a diagnosis is on clear after clinical assessment, and that antibiotic should be prescribed based on the results. Immediate antibiotic treatment should be offered if the CRP level is more than 100 mg/L and delayed prescription should be considered at the levels between 20 mg/L to 100 mg/L and is not recommended for CRP levels less than 20 mg/L [2].
CRP is an acute-phase protein produced by the liver following infection or injury. Measuring CRP in people presenting a suspected lower respiratory tract infections house differentiate viral and self-limiting infections from more serious bacterial infections that need antibiotics. Currently, CRP levels can be measured using a number of point-of-care devices. NICE recognises the following CE marked devices that all quantify CRP levels in the blood: Afinion AS 100 (Alere), AQT90 Flex (Radiometer Medical ApS), i-CHROMA™ (Boditech Med), NycoCard Reader II (Alere), Smart analyser (Eurolyser Diagnostica), Quick Read Go and has published evaluations on the Afinion and QuikRead (Orion Diagnostics) [3,4].
Albumin is a protein that is present in the blood stream and filtered via the kidneys [5]. During normal functioning of the kidney, there is no loss of albumin via the kidneys, when there is damage to the kidneys as a result of a number of conditions such as high blood pressure, heart failure, liver cirrhosis or systemic lupus erythematosus, diabetes small amount of albumin are filtered from kidneys, which results in a condition called microalbuminuria [6,7]. A microalbuminuria (MAU) test is the estimation of microalbumin on a random urine sample, a urine sample collected over a 24 hour period or a urine sample collected over a specific period of time, such as 4 hours or overnight [8]. MAU levels can also be measured using a variety of POCT devices. The Alere Afinion, Nyocard, Eurolyser, DCA Vantage, Hemocue, Clinitek Status, Immunodip and the i- CHROMA™ are all such devices [9].
The Boditech i-CHROMA™ is a novel fluorescence-based immunoassay that provides quantitative analysis of a range of tests in urine, serum, plasma or whole blood. The reaction is performed using disposable cartridges and the result is measured using the portable i-CHROMA™ Reader. It measures the following tests: Troponin, D-Dimer, CK-MB, Myoglobin, HsCRP, PSA, AFP, CEA, iFOB, HbA1C, Microalbumin, Cortisol, hCG, B-HCG, FSH LH, TSH, T3, T4, Prolactin, Testosterone, FSH, Progesterone, RF (IgM), ASO, CRP, HsCRP, procalcitonin, ferritin and cystatin C. Recently, studies have been demonstrated the performance of the Boditech i-CHROMA™ with regards to its PSA, HCG, LH and FSH estimation compared with routine laboratory methods [10,11]. The aim of this study was to evaluate the performance of the Boditech i-CHROMA™ CRP and MAU methods for the analysis of serum and urine against a traditional laboratory method, the Abbott Architect Ci8200 CRP method.
Materials and Methods
CRP serum samples (n=44) and MAU urine samples (n=25) used for this study were surplus, anonymised serum samples that were received by the laboratory for the routine measurement of CRP and MAU using the Abbott Architect Ci8200 at the Homerton University Hospital. The samples were also analysed using the i-CHROMA™ CRP and MAU method. The samples were run in one single run with no duplicates. The default result unit of the Boditech i-CHROMA™ CRP test is displayed as mg/L. The working range and detection limit of the test system are 2.5 to 300 mg/L and 2.5 mg/L. The default result unit Boditech i-CHROMA™ MAU test is displayed as mg/L from i-CHROMATM Reader. The working range is 2 to 300 mg/L.
The results obtained using the Abbott Architect Ci8200 and the Boditech i-CHROMA™ CRP and MAU methods were compared through Correlation Coefficients, Bland-Altman plots and paired T test.
i-CHROMA™ CRP and MAU principle
i-CHROMA™ CRP and MAU use a sandwich immunodetection method, such that by mixing the detection buffer with the blood specimen in the test vial, the fluorescence labelled detector anti-CRP or anti-MAU antibody in the buffer binds to the CRP or MAU antigen in the blood specimen. The sample mixture is loaded and migrates on the matrix of the test cartridge; the complexes of the detector antibody and CRP or MAU are captured to the anti-CRP or MAU sandwich pair antibody that has been immobilized on the test matrix. The fluorescence intensities are converted into a CRP or MAU concentration calculated by pre-programmed calibration process. The result of the tests is displayed on the reader as ng/mL for CRP and mg/L for microalbumin.
CRP test procedure:
• Draw whole sample using the sample collector.
• Assemble the sample collector with detection buffer tube.
• Shake the tube up and down 10 times or more.
• Discard 2 drops.
• Apply 2 drops to the test cartridge.
• Insert the test cartridge into reader and press ‘select’.
• Wait 3 minutes.
• Read result.
MAU test procedure:
• Draw 10 ul of test sample.
• Add it into the detection buffer tube
• Shake the tube up and down 10 times or more.
• Draw 75 μl of sample mixture.
• Load the sample mixture onto the test cartridge.
• Incubate for 12 minutes.
• Insert the test cartridge into reader and Press ‘select’.
• Read result.
Results
CRP– Evaluation of correlation
The data showed that overall the Boditech i-CHROMA™ CRP method showed good correlation with the Abbott Architect CRP method (r2=0.905). A regression analysis between i- CHROMA (y axis) and Ci8200 (x axis) yielded a slope of 0.74 and a y intercept of 13.96 mg/L (Figure 1).

Figure 1: Correlation of the i-CHROMA™ vs the Abbott Architect Ci8200 for the measurement of CRP (n=44).
CRP– Evaluation of bias
Results show that the Bland-Altman plots revealed little disagreement between the Boditech i-CHROMA™ CRP method and the Abbott Architect Ci8200 method. There was a positive bias relative of -8.1 mg/L (-9.1%) to the Abbott Architect method (Figure 2). There was no statistical difference between the estimations of both methods (p=0.594).
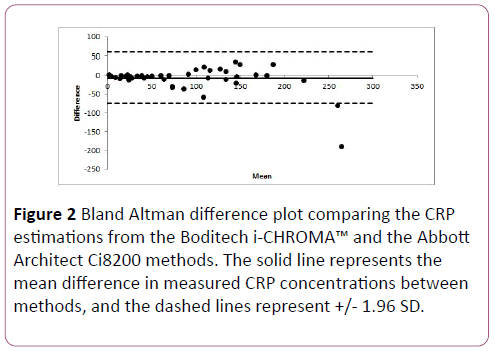
Figure 2: Bland Altman difference plot comparing the CRP estimations from the Boditech i-CHROMA™ and the Abbott Architect Ci8200 methods. The solid line represents the mean difference in measured CRP concentrations between methods, and the dashed lines represent +/- 1.96 SD.
CRP clinical evaluation
The results of the CRP estimations from the Boditech Med i- CHROMA™ and Abbott Architect Ci8200 methods using the assessment (recommended by NICE) to inform treatment options: Low CRP (<2 ng/mL), Intermediate CRP (2 to 10 ng/ mL), High CRP (>10 ng/mL). The Boditech i-CHROMA™ method identified 0 samples with low CRPs compared with 1 samples identified by the Abbott Architect Ci8200 method. The Boditech i-CHROMA™ method identified 5 samples with intermediate CRPs compared with 2 sample identified by the Abbott Architect Ci8200 method. The Boditech i-CHROMA™ method identified 39 samples with high CRPs compared with 41 samples identified by the Abbott Architect Ci8200 method (Table 1).
| Analyte |
Low CRP |
Intermediate CRP |
High CRP |
| Boditech i-CHROMA™ CRP Method |
0 |
5 |
39 |
| Abbott Architect Ci8200 CRP Method |
1 |
2 |
41 |
Table 1: Analysis of i-CHROMA™ CRP estimations using the Abbott Architect as standard, serum n=44.
MAU– Evaluation of correlation
The data showed that overall the Boditech i-CHROMA™ MAU method showed good correlation with the Abbott Architect Ci8200 CRP method (r2=0.987). A regression analysis between Boditech i-CHROMA™ (y axis) and Abbott Architect Ci8200 (x axis) yielded a slope of 0.59 and a y intercept of 15.93 mg/L (Figure 3).
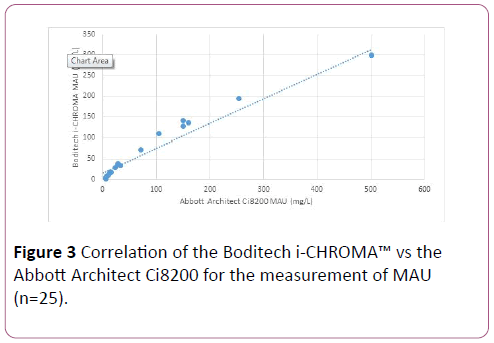
Figure 3: Correlation of the Boditech i-CHROMA™ vs the Abbott Architect Ci8200 for the measurement of MAU (n=25).
MAU– Evaluation of bias
Results show that the Boditech i-CHROMA™ method typically had a negative bias relative of -27.88 mg/L (-27.88%) to the Abbott Architect Ci8200 method (Figure 4). There was no statistical difference between the estimations of both methods (p=0.410).
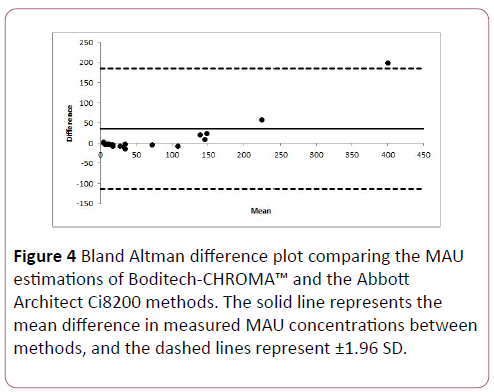
Figure 4: Bland Altman difference plot comparing the MAU estimations of Boditech-CHROMA™ and the Abbott Architect Ci8200 methods. The solid line represents the mean difference in measured MAU concentrations between methods, and the dashed lines represent ±1.96 SD.
MAU- Clinical evaluation
The results of the CRP estimations from the Boditech i- CHROMA™ and Abbott Architect Ci8200 methods using the assessment (recommended by NICE) to inform treatment options: Low MAU (<30 mg/L), Normal MAU (30 to 300 mg/L), High MAU (>300 mg/L). The Boditech i-CHROMA™ method identified 14 samples with low MAU’s compared with 12 samples identified by the Abbott Architect Ci8200 method. The Boditech i-CHROMA™ method identified 9 samples with normal MAU’s compared with 7 samples identified by the Abbott Architect method. The Boditech i-CHROMA™ method identified 4 samples with high MAU’s compared with 4 samples identified by the Abbott Architect Ci8200 method (Table 2).
| Variables |
Low |
Normal |
High |
| i-CHROMA™ MAU Method |
12 |
9 |
4 |
| Abbott Architect Ci8200 MAU Method |
14 |
7 |
4 |
Table 2: Analysis of i-CHROMA™ MAU estimations using the Abbott Architect as standard, urine n=25.
Discussion
Our results show that overall the correlation of the i- CHROMA™ CRP results against the Abbott Architect Ci8200 CRP method was very good (r2=0.905). The i-CHROMA™ CRP method showed a slight negative bias (-9.05% bias difference) when compared with the Abbott Architect Ci8200 CRP method. Previous studies have also shown the i-CHROMA™ CRP method to be a good method for the quantification of CRP. For example, when compared to the Beckman Coulter analyser, the i-CHROMA™ CRP method showed a good correlation (r2=0.84) and as a result was recommended as an attractive instrument for medical centers [12]. Minnard et al. also showed in two comparative studies comparing the i- CHROMA™ method with some common CRP POCTs that the i- CHROMA™ CRP method is a good method for measuring CRP values [13,14]. One study by Brouwer et al. also confirmed the i-CHROMA™ CRP method a good CRP POC test [15]. In addition, Oh et al. confirmed that the i-CHROMA™ CRP method was a suitable one when compared with other wellknown CRP assays [16]. There are several studies conducted with CRP point of care testing in adults to guide antibiotic prescribing in respiratory tract infections, which we would evaluate. The test methodology used the following test result ranges (recommended by NICE) to inform treatment options: Low CRP (<20 mg/L) rules out need for antibiotics, Intermediate; (CRP 20 to 100 mg/L) use clinical judgement to decide to need for antibiotics; High CRP (>100 mg/L) rules in need for antibiotics. From the tests, the Boditech i-CHROMA™ method estimations would have recommended the need for the use of antibiotics in 39 (95%) of the samples compared to 41 samples by the Abbott Architect Ci8200 method.
The ability to screen urine microalbumin in patients with diabetes is important. A number of POCT devices for testing MAU have been evaluated. Lloyd et al assessed the performance of the Hemocue and Clinitek MAU POCT methods and found both the Hemocue and Clinitek to have a good correlation against traditional laboratory quantification [17]. Omoruyi et al. showed that 2 POCT devices (Afinion and DCA Vantage) were in good agreement with quantitative laboratory methods [18]. This is the first comparison study of the Boditech i-CHROMA™ MAU method and a traditional laboratory method, the Abbott Architect Ci8200 MAU method. The results show that overall the correlation of the Boditech i- CHROMA™ MAU method against the Abbott Architect Ci8200 CRP method was very good (r2=0.987). Although there was a `negative bias (-27.88% bias difference), using the following test result ranges (recommended by NICE) to inform treatment options: Low MAU (<30 mg/L) which rules out need for further investigation as this indicates a normal amount of MAU, Normal MAU (30 to 300 mg/L) would require clinical judgment to diagnose microalbuminuria and High MAU (>300 mg/L) which suggests clinical albuminuria. The Boditech i-CHROMA™ method detected the same number (4 out 4) of high MAU results as the Abbott Architect Ci8200 MAU method.
Conclusion
The Boditech i-CHROMA™ POCT CRP and MAU methods showed very good correlation with a traditional laboratory method and therefore provides a reliable measurement of serum CRP and urine MAU samples.
20536
References
- Afinion A (2016) CRP for C-reactive protein testing in primary care. Guidance and Guidelines. NICE (National Institute for Health and Care Excellence), UK.
- National Institute for Health and Care Excellence (2017) Pneumonia in adults: Diagnosis and management. Guidance and guidelines, UK.
- Cals J, Schot M, De Jong S, Dinant G, Hopstaken R (2010) Point-of-care C-reactive protein testing and antibiotic prescribing for respiratory tract infections: A randomized controlled trial. Ann Fam Med 8: 124-133.
- Andreeva E, Melbye H (2014) Usefulness of C-reactive protein testing in acute cough/respiratory tract infection: an open cluster-randomized clinical trial with C-reactive protein testing in the intervention group. BMC Fam Pract 15: 80.
- Doumas B, Ard Watson W, Biggs H (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta 31: 87-96.
- Mogensen CE, Christnesen CK (1984) Predicting diabetic nephropathy in insulin dependent diabetes. New Eng J Med 311: 89-93.
- Viberti GC, Hill RD, Jarrett RJ (1982) Microalbuminuria as a predictor of clinical nephropathy in insulin dependent diabetes mellitus. Lancet 1: 1430-1432.
- Mathiesesen ER, Ronn B, Jensen T, Deckert T (1990) Relationship between blood pressure and urinary excretion in the development of microalbuminurea. Diabetes 39: 245-249.
- NHS National Health Service (2017) Point of care devices for the measurement of HbA1c and low concentration albumin in urine. London, NHS, 2009. UK.
- Bolodeoku J, Bains S, Chand V, Bacon R, Weir P, et al. (2017) A screening evaluation of the Point of Care Test (POCT) i-CHROMA prostate specific Antigen (PSA) assay method in the community. Point of Care. J Near Patient Test Technol 16: 93–96.
- Bolodeoku J, Bains S, Pinkney S, Coker O, Fakokunde A (2017) Comparison of the point of care test (POCT), i-CHROMA human chorionic gonadotrophin (HCG), leutinizing hormone (LH) and follicle stimulating hormone (FSH) methods with the other laboratory methods in the Randox International Quality Assessment Scheme (RIQAS). Clin Obstet Gynacol Reprod Med 3: 1-7.
- Ciftci I, Koroglu M, Karakece E (2014) Comparison of novel and familiar commercial kits for detection of C-reactive protein levels. World J Microbiol Biotechnol 30: 2295-2298.
- Minnaard M, Van De Pol A, Broekhuizen B, Verheij T, Hopstaken R, et al. (2013) Analytical performance, agreement and user-friendliness of five C-reactive protein point-of-care tests. Scand J Clin Lab Investig 73: 627-634.
- Minnaard M, Van De Pol A, De Grootr J, De wit N (2015) The added diagnostic value of five different C-reactive protein point-of-care test devices in detecting pneumonia in primary care: A nested case-control study. Scand J Clinical Lab Investi 75: 291-295.
- Brouwer N, Van Pelt J (2015) Validation and evaluation of eight commercially available point of care CRP methods. Clinical Chimica Acta 439: 195-201.
- Oh S, Moon J, Park S, Jang H, Kim J, et al. (2005) Evaluation of fluorescence hs-CRP immunoassay for point-of-care testing. Clinica Chimica Acta 356: 172-177.
- Lloyd M, Kuyl J, Jaarsyeld H (2011) Evaluation of point-of-care tests for detecting microalbuminuria in diabetic patients. South Afr Fam Pract 53: 281-286.
- Omoruyi FO, Mustafa GM, Okorodudu AO, Petersen JR (2012) Evaluation of the performance of urine albumin, creatinine and albumin-creatinine ratio assay of two POCT analyzers relative to a central laboratory method. Clinica Chimica Acta 413: 625-629.










