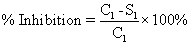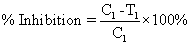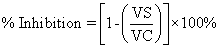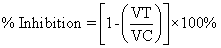Keywords
|
| Mangifera indica; Indomethacin; Anti-inflammatory; Carrageenan; Mangiferin; Granuloma pouch |
Introduction
|
| Inflammation is defined as local response of living tissue to injury due to any agent. Inflammation manifests usually in form of painful swelling associated with some changes in skin covering the site [1]. Inflammation can be classified as either acute or chronic.Acute inflammationis the initial response of the body to harmful stimuli and is achieved by the increased movement of plasma and leukocytes from the blood into the injured tissues. A cascade of biochemical events propagates and matures the inflammatory response, involving the local vascular system, the immune system, and various cells within the injured tissue. Prolonged inflammation, known as chronic inflammation, leads to a progressive shift in the type of cells which are present at the site of inflammation and is characterized by simultaneous destruction and healing of the tissue from the inflammatory process [2]. |
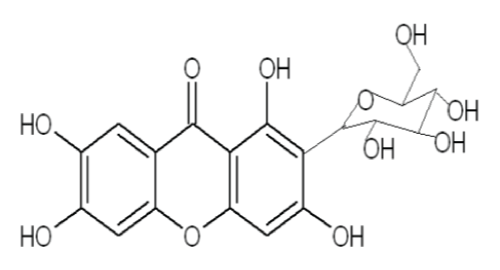
| Mangiferin, C19H18O11, a glucoxanthone (1, 3, 6, 7-tetrahydroxyxanthone-C2-β-Dglucoside) (Figure 1) has been reported to be present in various parts of M. indica (Anacardiaceae) and was encountered for the first time by Wiechowski. The conclusive structure of mangiferin has been established as 2-C-β-D-glucopyranosyl- 1, 3, 6, 7-tetrahydroxyxanthone. Mangiferin occurs widely among angiosperms and has also been identified in ferns. |
| Mangiferin has been traditionally used as anti-inflammatory, analgesic, antioxidant, immunomodulator and in obesity treatment, particularly for diabetes type II. In Cuba and Sri Lanka it is sold under brand names Vimang® and Salaretin®, respectively. An extensive and recent literature survey has revealed that mangiferin has been isolated from various parts of M. indica and different methods have been employed to establish its chemical structure as- well as its pharmacological activities through biological screening procedures [3]. |
Experimental collection of the plant material
|
|
Instrumentation
|
| Soxhlet extractor consisting of condenser, extraction chamber, thimble, siphon arm, boiling flask, heating mantle, pipes for flow of water (Figure 2). Buchner funnel and Whattman filter paper for filtration, glass rod, and beaker. |
|
Reagent
|
| Methanol (CH3OH) as solvent. |
|
Procedure
|
| The mango leaves were collected and shade dried for 21 days and then dried leaves were chopped and crushed by the mixer grinder and passed through sieves, to made fine free flowing powder. After that the powdered leaves (82.50 gm) were soxhlet extracted for atleast 36 hr. with the solvent methanol (methayl alcohol). Then the extracted material was filtered by Buchner funnel and was kept in the desscicator to evaporate the moisture. Crystals were formed and isolated, mainly it was a clay type sticky extract. The extraction was done in different segments and it was a time consuming phase of this experiment. The temerature of the extaction remained in between 45°C-55°C. |
|
Calculations
|
| Here, the quantity obtained of the extract was 7.50 gm after completion of the whole extraction process. So, here theoretical yield (TY)=82.50 gm and practical yield (PY)=7.50 gm. So, percentage (%) yield=[PY/TY] × 100%=[7.50/82.50] × 100%=9.09% (% yield of the mango leaf methanolic extract) [4]. |
|
Instrumentation
|
| Cotton pellets (each weighing 30 ± 5 mg), Digital weighing balance, Hot air oven, Disposable syringe (2 ml), Tuberculin needle (26.5 gauze), Surgical thread (thin), Surgical bend needle (small), Distilled water, Surgical blade (no. 11), Surgical blade holder (no. 04), Forceps, Arterial forcep, Beaker, Glass rod, Feeding needle for rats, Filter paper, Tissue paper, Pipette (10 ml). |
|
Reagent
|
| Thiopentone sodium inj. (for general anesthesia; diluted in sterile water for inj.), Methanolic extract of mango leaf (test solution; diluted in distilled water), Normal saline solution (NS; control solution), Indomethacin capsule (25 mg; standard solution; diluted in distilled water), Anesthetic ether (for general anesthesia), Picric acid solution (for marking the animals), Savlon antiseptic liquid, Betadine (povidone iodine) ointment. |
|
Animal
|
| Wistar rats (body wt. 100-150 gm; total no. 30; sex: male). |
|
Procedure
|
| At first the animals were weighed using the digital weighing balance. Then they were marked by the picric acid solution to distinguish them according to their body weight. After that the animals were grouped as control (no. 6), test (no. 18) and standard (no. 6) respectively. The groups were fasted at a period of 24 hr prior to the study. The Sterile cotton pellets each weighing 30 ± 5 mg were prepared and sterilized in a hot air oven at 123°C for 3 hr. Each animal was placed under light with general anesthesia by injecting Thiopentone sodium (40 mg/kg bw) diluted with the vehicle sterile water for injection and inhalation of Anesthetic ether. Then the cotton pellets were subcutaneously implanted in the number of one each to each animal in the groin region under aseptic conditions. The drugs were administered orally by feeding needle for seven days starting from the day of implantation of the pellets. The three animals in the control group were given NS solution and the three animals in the standard group were given Indomethacin solution at a dose of 10 mg/kg body weight orally. The test group was given methanolic extract of mango leaf solution orally in the doses of 200 mg/kg bw (6 animals), 500 mg/kg bw (6 animals), 1000 mg/kg bw (6 animals) [Here, bw=body weight]. All the animals had free access to drinking water and food. On the 8th day, all the animals were sacrificed and the implanted cotton pellets were recovered, cleaned of surrounding tissues, and blotted with filter paper. These cleaned pellets were weighed and dried in a hot air oven overnight at 70°C and the dry weights were noted. Percentage inhibition of Granuloma Pouch in rats was calculated using the following formula: |
| % Inhibition={(Control-Test)/Control} × 100% |
|
Carrageenan induced paw edema technique (acute inflammation)
|
|
Instrumentation:
|
| Digital weighing balance, Disposable syringe (2 ml), Tuberculin needle (26.5 gauze), Distilled water, Beaker, Glass rod, Feeding needle for rats, Pipette (10 ml), Plythesmometer (INCO, Ugo Basile, Italy Model 7150). |
|
Reagent:
|
| Carrageenan (as phlogistic agent), Methanolic extract of mango leaf (test solution; diluted in distilled water), Normal saline solution (NS; control solution), Indomethacin capsule (25 mg; standard solution; diluted in distilled water), Picric acid solution (for marking the animals). |
|
Animal:
|
| Wistar rats (body wt. 100-150 gm; total no. 30; sex: male). |
|
Procedure:
|
| At first the animals were weighed using the digital weighing balance. Then they were marked by the picric acid solution to distinguish them according to their body weight. After that the animals were grouped as control (no. 6), test (no. 18) and standard (no. 6) respectively. The groups were fasted at a period of 24 hr prior to the study. The test compound (the extract) was administered to the animals in the test group at the dose of 200, 500 and 1000 mg/kg bw by oral route to each 6 animals respectively by the feeding needle. [Here, bw=body weight] Animals in the standard group received Indomethacin at the dose of 10 mg/kg bw by oral route. Rats in control group received NS solution or the vehicle solution without drugs. One hour after drugs administration, rats in all groups were challenged with 0.1 ml of 1% (w/v) carrageenan in sub-planter region of left hind paw. A zero hour paw volume was measured for the rats using Plythesmometer before the administration of carrageenan for all groups. The volumes of edema of the carrageenan injected paws were measured at 1 hr interval for 4 hr. In the above model, % inhibition of edema was calculated as follows: % Inhibition of edema=(1-VT/VC) × 100% Where, VT is the inflammatory increase in paw volume of the rats of treated groups. VC is the inflammatory increase in paw volume of the rats of control groups. |
Results and Discussion
|
| % Inhibition was calculated as - |
| In case of Standard 1 (S1): |
| % Inhibition={(C1-S1)/C1} × 100%={(116-57.5)/116} × 100%=50.43%. |
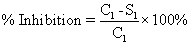 |
| In case of Test 1 (T1): |
| % Inhibition={(C1-T1)/C1} × 100%={(116-101)/116} × 100%=12.93%. |
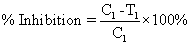 |
| % Inhibition was calculated as – |
| In case of Standard (S): |
| % Inhibition=[1-(VS/VT)] × 100%=[1-(0.15/0.15)] × 100%=0% (After 1st hr) |
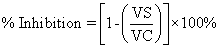 |
| In case of Test (T): |
| % Inhibition=[1-(VT/VC)] × 100%=[1-(0.15/0.15)] × 100%=0% (After 1st hr) |
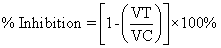 |
| The present study shows that aqueous (methanolic) leaf extract (test) of Mangifera indica (family: Anacardiaceae) possesses antiinflammatory activity in both cotton pellet granuloma technique and carrageenan induced acute rat paw edema technique (Tables 1 and 2). The activity profile of extract at 1000 mg/kg closely resembled to that of Indomethacin (standard drug). Carrageenan induced paw edema was taken as a prototype of exudative phase of acute inflammation. Inflammatory stimuli microbes, chemicals and necrosed cells activate the different mediator systems through a common trigger mechanism. The development of carrageenan induced edema is believed to be biphasic. The early phase is attributed to the release of histamine and serotonin and the delayed phase is sustained by the leukotrienes and prostaglandins. To further verify the anti-inflammatory activity of the extracts and its effects on the proliferative phase of inflammation, cotton pellet granuloma formation was used. The aqueous (methanolic) leaf extract at a dose level of 200, 500 and 1000 mg/kg showed a significant inhibitory effect on granuloma formation. This study revealed that the methanolic extract was active against the inflammation induced by a foreign body. Here also the activity profile of extract at 1000 mg/kg closely resembled to that of Indomethacin [5]. In case of cotton pellet granuloma technique: The % Inhibition of granuloma pouch-In case of standard (Indomethacin; 10 mg/kg) is 50.65%. In case of test extract (200 mg/kg) is 10.08%. In case of test extract (500 mg/kg) is 28.45%. In case of test extract (1000 mg/kg) is 48.72%. In case of carrageenan induced acute rat paw edema technique: The % Inhibition of edema- In case of standard (Indomethacin; 10 mg/kg) is 37.5%. In case of test extract (200 mg/kg) is 7.5%. In case of test extract (500 mg/kg) is 13.4%. In case of test extract (1000 mg/kg) is 36.1%. |
| Carrageenan induced paw inflammation has been accepted as a useful phlogistic tool for investigating systemic anti-inflammatory agents.Acute inflammation is produced when water and plasma increases in tissues during arachidonic acid metabolism via cyclooxygenase and lipo-oxygenase enzyme pathways. It has two phases: 1st phase (begins immediately after Carrageenan injection and lasts for 1 hr) is characterized by release of histamine and serotonin; and 2nd phase (begins after 1 hr and lasts for 4 hr) is characterized by bradykinin release by prostaglandin mediator pathways. In order to assess its efficacy against proliferative phase of inflammation, in which tissue degeneration and fibrosis occur, cotton pellet granuloma test was employed. During repair process of inflammation, there is proliferation of macrophages, neutrophils, fibroblasts and multiplication of small blood vessels, which are basic sources for forming a highly vascularised reddish mass termed as granulation tissue. In case of cotton pellet induced granuloma, - there was significant reduction in granular tissue formation. This result is in- confirmation with anti-proliferative activity of extract. Thus, in the light of results of carrageenan induced paw edema and cotton pellet granuloma Mangifera indica leaf powder for systemic application in inflammatory conditions can be explained [6] (Plots I-IV). |
Conclusions
|
| In view of our interest in the chemical constituents of indigenous medicinal plants, the chemical examination of the dried leaves of Mangifera indica (family: Anacardiaceae) has now been undertaken. The present pharmacological study was undertaken to evaluate the possible anti-inflammatory properties of the aqueous methanolic leaf extract from Mangifera indica. The study was therefore aimed at investigation of the anti-inflammatory activity of leaf extract with a view to justifying the use of the plant in the treatment of inflammatory diseases [1]. |
| Therefore it is worthwhile to conduct detailed studies in order to explore the full potential of this plant in reducing inflammation in humans from the point of view of cost and availability for people at all socioeconomic levels [2]. |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|