Keywords
|
| Anti-fungal; Optimization; Cubosomes; Candidiasis; Topical drug delivery; Fluconazole |
Introduction
|
| Fluconazole is a hydrophilic bis-triazole compound having broad spectrum antifungal activity [1,2]. It has been extensively used as a first line agent to treat various fungal infections such as vulvovaginal candidiais, oropharyngeal candidiasis, mucosal leishmaniasis, visceral leishmaniasis and dermatomycosis. Oral and parenteral administration of the drug is associated with various side effects including headache, nausea, vomiting and abdominal pain [3]. Further retinal, hepatic and renal toxicity was also observed in patients on high and prolonged dose of Fluconazole [4]. Moreover, invasive parenteral delivery leads to poor patient compliance [5]. Literature review has also revealed less absorption of the drug through skin as the drug is more hydrophilic as compared to other drugs of its class with experimental log P value within the range of 0.45-0.5. |
| Salerno et al. investigated different topical dosage forms of fluconazole i.e., emulsions, emulgels, lipogels and thickened microemulsion based hydrogel for efficient loading and delivery of the drug to the skin [6]. As a result of the study it was found that microemulsion based system loaded and delivered the drug more efficiently than other dosage forms. Patel et al. prepared and evaluated the effect of formulation variables on microemulsion containing fluconazole to treat topical fungal infections against Candida albicans [7]. It was observed that by altering the composition of formulation variables like lipid, water, stabilizer suitable system can be developed which can offer desirable performance. Thus, it becomes clear that cubosomal system can serve as an ideal drug delivery system for drugs with poor loading and permeability for the treatment of topical fungal infections [8]. |
| Cubosomes are the self- assembled invert [9,10] bicontinuous cubic nanoparticles (100-500 nm) composed of lipid layers, separating nonintersecting water channels [11]. The unique cubic particles because of their internal surface have been reported to enhance the drug pay load. These unique nanoparticles have extensively been studied in research with particular focus on cosmeceuticals [12,13]. These liquid cubic crystals have been envisioned as future drug delivery vehicles [14] since their constituents are biocompatible, bioadhesive, nontoxic, non-immunogenic and cost efficient. Unique structure of cubosomes can incorporate wide varieties of drugs (hydrophilic, lipophilic and amphiphilic) with good pay load. In recent years, cubosomes have been studied as potential carrier for dermal, ocular, oral, nasal, periodontal, buccal, and vaginal drug delivery [11,14]. Various investigations have also indicated towards the sustained or prolonged drug delivery abilities of cubosomes and consistent release of drug through the cubic vesicles. Cubosomes are also capable to protect the labile bioactives such as proteins, peptides and genes while improving their therapeutic effectiveness [15]. The aim of the presented work was formulation development and evaluation of cubosomal gel loaded with fluconazole for topical drug delivery with high drug payload for improved efficiency. The system was developed by keeping the objectives to enhance the permeation and accumulation of drug in the skin. In an attempt to achieve the above targets, cubosomal gel loaded with fluconazole was developed and evaluated for the effectiveness. |
Materials and Methods
|
|
Materials
|
| Rylo MG™ Glyceryl mono-oleate (G.M.O) was obtained as a free gift from Danisco (Grinsted, Denmark). The purity of the sample complied with USP-NF analytical specifications. Fluconazole (99.8% pure) was obtained as a free gift from Symed Lab Ltd. (SREC arcade, Andhra Pradesh, India). PEO-PPO-PEO Triblock copolymer, Poloxamer 407 was purchased from SD Fine Chem. Ltd. (Mumbai, India). All the other chemicals were of laboratory grade and were obtained from Loba Chemie Pvt. Ltd (Mumbai, India). The goat’s skin for studying permeation was obtained from the local slaughter house. |
|
Methods
|
|
Method validation for fluconazole in pH 6.4 phosphate buffer by UV-visible spectrophotometer:
|
| The calibration curve for fluconazole in pH 6.4 phosphate buffer was plotted by using double beam UV-visible spectrophotometer (Shimadzu Co. Ltd., Japan) [16]. The absorbance maximum (λmax) was noted for fluconazole. The linear regression analysis was carried out for the concentration range of 50-400 μg/ml in triplicate. Accuracy was determined from mean recovery obtained from three different concentration level of fluconazole solution. Intra and inter day precision studies were carried out at three different time points on same and different days respectively. The robustness of method along with limit of detection (LOD) and limit of quantification (LOQ) of analyte in sample was determined with suitable precision and accuracy [17]. |
|
Preformulation studies:
|
| Compatibility study was carried out for pure drug, excipients and drug: excipient mixture in ratio of 1:1. The mixtures were placed in glass containers and stored at temperature 50?C and 60?Cwith 75% RH as per ICH guidelines for stress testing [18]. Physical observation of mixtures and pure samples were made on 0th and 15th day for change in colour, appearance, state and lump formation along with recording FTIR spectrum to ensure chemical compatibility of mixture. The effect of the excipients on the major absorption peaks of fluconazole was observed to determine the compatibility of the drug and excipients. |
| The solubility study of fluconazole was carried out in different solutions used during study [19]. The solutions of different pH viz., (pH 1.2) 0.1N HCl, pH 4.5 acetate buffer, pH 6.4 phosphate buffer, pH 7.4 phosphate buffer and lipid solution (GMO) in ethanol were prepared. Ten ml of each buffer was transferred to different containers to which, known excess amount of drug was added to saturate the solution. The drug solutions were maintained at 32 ±2?C on a water bath shaker (Raj Analytical Services, India) by shaking at 80 horizontal strokes per minute for 72 hrs. The samples were analysed for fluconazole content using a UV-spectrophotometer at λmax 261 nm after suitable dilution. |
|
Screening studies
|
|
Components and preparation technique for cubosomal dispersion:
|
| Screening studies were carried out to select ingredients and their ratios required to prepare cubosomal formulations. Another objective was to check the effect of lipid: polymer ratio on the properties of cubosomes. GMO and soyalecithin were used as lipids whereas; poloxamer 407 and poloxamer 188 were used as polymers. Total nine batches with low, medium and high ranges of lipid: Polymer were prepared (Table 1). The effect of ratio was analysed by observing the shape and size of the cubic particles on optical microscope at 100 X magnification. Cubosomes were prepared as per the method reported previously by Yang et al. GMO and fluconazole were weighed accurately and mixed in different ratios by melting the lipid in water bath maintained at 60°C. Excess amount of distilled water (2 ml) preheated at 60°C temperature was added into the molten mixture while stirring. The mixture was then kept at room temperature (25°C) until a clear gel was formed. Weighed amount of Poloxamer 407 was dissolved in distilled water and added to drug-lipid mixture with stirring. A gel like system was formed. The gel system was then sonicated at 37 ± 2°C for 60 minutes. The prepared system was then homogenized by passing the system through a syringe fitted with 21 gauge needle repeatedly for twenty cycles [20,21]. |
|
Formulation development
|
|
Preparation of optimized formulation by DoE technique:
|
| Different batches of drug loaded cubosomes were prepared by using screened out formulation components and their ratios at different levels (Table 2). A Central composite design (CCD) was selected for three factors (X1, X2 and X3) X1: Drug, X2: Lipid and X3: polymer analysed at three levels w.r.t the response variables Y1: Entrapment efficiency and Y2: Permeability at 6th hour. Preparation technique mentioned in the above section of “Components and preparation technique for cubosomal dispersion” was used to prepare all the formulations. The formulations were designed and optimized by applying design of Experiment (DoE) techniques with the help of a software program (Design Expert- 8.0.1). Table 3 shows formulation components and their quantities used to prepare 20 formulations including 5 replicates. |
|
Characterization and evaluation of cubosomes
|
|
Particle size distribution and morphological analysis:
|
| Particle size was observed by Photon Correlation Spectroscopy (PCS) using Zeta sizer (Malvern Instruments Ltd. UK) [22] for the optimized cubosomal formulation, which was prepared by ultrasonication and homogenization. The morphology of the cubosomal dispersion was analysed by Transmission electron microscopy. A drop of a sample (cubosomal dispersion) was placed onto a carbon-coated grid and allowed to dry [23]. The grid containing the sample was observed under the transmission electron microscope (Hitachi Scientific Instruments, Tokyo, Japan) with an accelerating voltage of 80 kV. The images were then obtained after focusing the microscope with different magnifications of 120000-400000X. |
|
Drug entrapment efficiency:
|
| The entrapment efficiency of prepared cubosomal formulations was observed by centrifugation method [24]. Cubosomes were centrifuged (REMI Electrotechnik Pvt Ltd. vasai, Mumbai, India) at 20000 rpm for 1 hour at controlled temperature. Above phase obtained as supernatant containing un-entrapped fluconazole was separated and measured by UV spectrophotometer at λmax 261 nm against phosphate buffer (pH 6.4). The remaining entrapped drug in cubosomes was measured after rupturing the cubosomes using triton X. The amount of fluconazole entrapped in cubosomes was determined by calculating the entrapment efficiency as follows (equation 1): |
| EE% = ( At - Af / At X 100) (1) |
| Where At is total amount of fluconazole and Af is the amount of free fluconazole [25]. The entrapment efficiency was obtained by repeating the experiment in triplicate and the values were expressed as mean standard deviation. |
|
Preparation of Secondary vehicle/ gel for fluconazole loaded cubosomes:
|
| The Carbopol gels (0.2, 0.6, 1.0, 1.2 and 1.8%) were prepared by using appropriate amount of Carbopol® 934NF which was dispersed in water with constant stirring to prevent the lump formation [26]. Methyl paraben, propyl paraben and sodium benzoate were added as preservatives. The gels thus obtained were checked for their viscosity and other physical parameters. The cubosomes were made rheologicaly acceptable by incorporating them into 1% w/w carbopol 934 gel. To adjust the pH, triethanolamine was added with constant stirring. |
|
Evaluation of optimized cubosomal gel of fluconazole
|
|
Rheology and texture analysis:
|
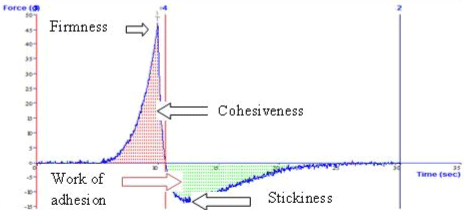
| Rheology of the gel was determined by using Rheometer (M/S Anton Paar, India) at 37°C. The plot of shear stress Vs shear rate was obtained. The line of equation was fitted to power law (y=Ktn) where K is the consistency and n represents the flow property of the system [27]. Various gel characteristics, i.e., firmness, stickiness, consistency and work of adhesion of a marketed and optimized cubosomal gel were determined by using texture analyser (Stable Micro Systems Ltd., Godalming, SurreyGU7 1YL, UK). |
|
Ex vivo skin permeation study:
|
| The Ex vivo skin permeation studies were carried out by using goat’s skin obtained from a slaughter house. The hair from the skin was removed by using the hair removal cream. The fatty layer of the skin was removed by keeping the skin in warm water at 60°C. After 5 minutes, the fatty layer of the skin was peeled off gently and the skin was kept in pH 7.4 phosphate buffer for saturation [28]. The skin was mounted on the receptor chamber or Franz diffusion cell with cross sectional area of 3.91 cm². The receptor compartment was filled with 25 ml of pH 6.4 phosphate buffer stirred with a magnetic bead. The cell was jacketed to maintain the temperature similar to skin i.e., 32 ± 0.5°C. Each batch of cubosomal gel was applied on the skin and 1 ml sample was withdrawn at different time intervals followed by replenishment with buffer to maintain sink conditions. The samples withdrawn were quantified spectrophotometrically at λmax 261 nm after suitable dilution. |
| In-vitro release kinetics of fluconazole from optimized cubosomal gel was analysed by mathematical modeling. The in vitro drug release was fitted to various release kinetics models viz., first-order, Higuchi, Hixson-Crowell cube root, Korsemeyer-Peppas and zero-order mathematical models [29]. Selection of a suitable release model was based on values of r2 (correlation coefficient), k (release constant) and n (diffusion exponent) obtained from curve fitting of release data. |
|
In vitro anti-fungal study
|
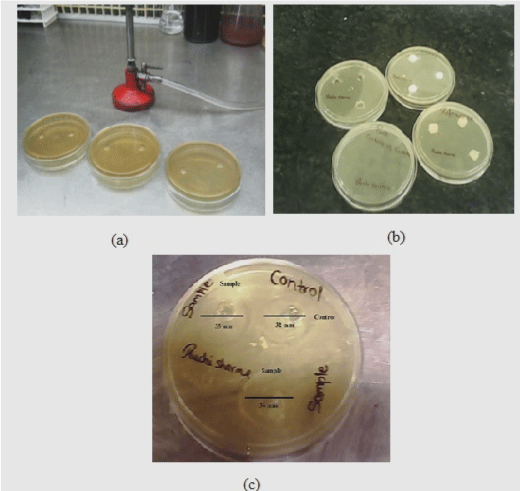
| The in vitro antifungal activity of the prepared optimized cubosomal gel formulation was tested in a triplicate manner using agar cup method against Candida albican strain [7]. Cup of 10 mm in diameter were cut aseptically under laminar airflow (Microflow Pvt. Ltd, India) in Sabouraud dextrose agar after being inoculated with tested fungal suspension strain by spreading on the agar surface [30]. The cups were filled with (2 ml) of 25 μg/ml formulation and control (Flucose®gel) by sterile syringe. The plates were then incubated at 30°C for 24 hr. The zone of inhibition of each cup was then determined after incubation period by measuring the radius of each zone and was compared with a controlled formulation. |
Results and Discussion
|
|
Preformulation studies
|
|
Analytical method validation of fluconazole in pH 6.4 phosphate:
|
| The absorbance maximum (λmax) of fluconazole was found to be 261 nm in pH 6.4 phosphate buffer. The calibration curve was found to be linear for concentration range of 50-450 μg/ml at 261 nm with significant higher value of correlation coefficient, R2=0.999. The results of accuracy and precision displayed good reproducibility with RSD value below 2. The method was found to be accurate and precise and there was negligible variation in intraday and interday precision. The method was not influenced by the slight changes in the pH of the solutions so it was found to be robust. The LOD and LOQ were found to be 6.285 μg/ml and 19.04 μg/ml respectively. These results demonstrated that the method was sensitive enough to detect and quantify the drug in formulation and release samples. |
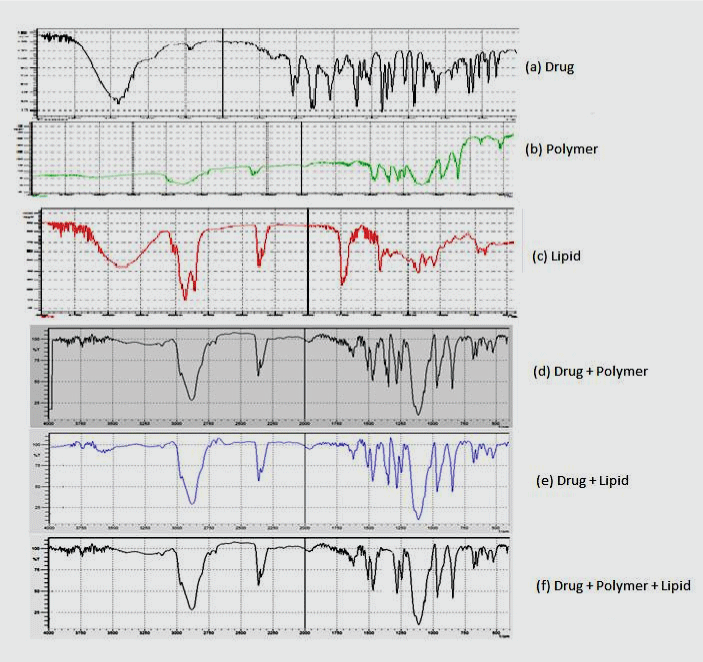
| From drug-excipient compatibility studies, it was observed that there was no change in colour and appearance of the drug excipients mixture, but a negligible change in state was observed due to melting of lipid on the 15th day. The chemical compatibility was assured by carrying out FTIR spectral analysis of drug with excipients [31] and by comparing the peaks (Figure 1). It was observed that the excipients did not interfere with the major absorption peaks of the drug indicating chemical compatibility between the drug and excipients. |
| The solubility profile showed maximum solubility of drug in pH 1.2 (0.1 N HCl) and minimum solubility in pH 6.4 (phosphate buffer). The pH solubility profile indicated that the solubility first reduces as the pH increases up to pH 6.4 then increases up to 11.2 μg/ml in pH 7.4. The solubility of the drug in GMO was found to be 10.28 μg/ml that indicated sparingly solubilizing behaviour of the drug in the lipid. It showed that the drug can be easily dispersed in the lipid system during preparation of cubosomes. |
|
Formulation development
|
| A Central Composite Response Surface Rotatable Design was employed to obtain 20 different factor combinations and their replicates. Entrapment efficiency (Y1) and permeability at 6th hour (Y2) were selected as the two response variables. The value of Factor of Design Space (FDS) was found to be 0.81 which means that fraction of design space was capable of predicting the true average below 1. |
| The formulations prepared according to the design were analysed by using Design Expert® ver 8.0.1 software package. The effect of formulation variables on the response variables were statistically evaluated by one way ANOVA at p<0.05 levels [32,33]. The design was evaluated by response surface method using following polynomial equation 2: |
| Y=β0 + β1X1+ β2X2 + β3X1X2 (2) |
| where, Y is the response variable, β0 the constant and β1, β2, β3 are the regression coefficients. X1 and X2 stand for the main effect, X1, X2 are the interaction terms and show how the response changes when two factors are simultaneously changed (Table 4). The quadratic model was selected on the basis of model p values, lack of fit test, adjusted R2 and predicted R2. Final polynomial equations for each response variable in terms of coded and actual factors were obtained with the constraints applied for each response (Table 5). |
|
Validation of optimized results
|
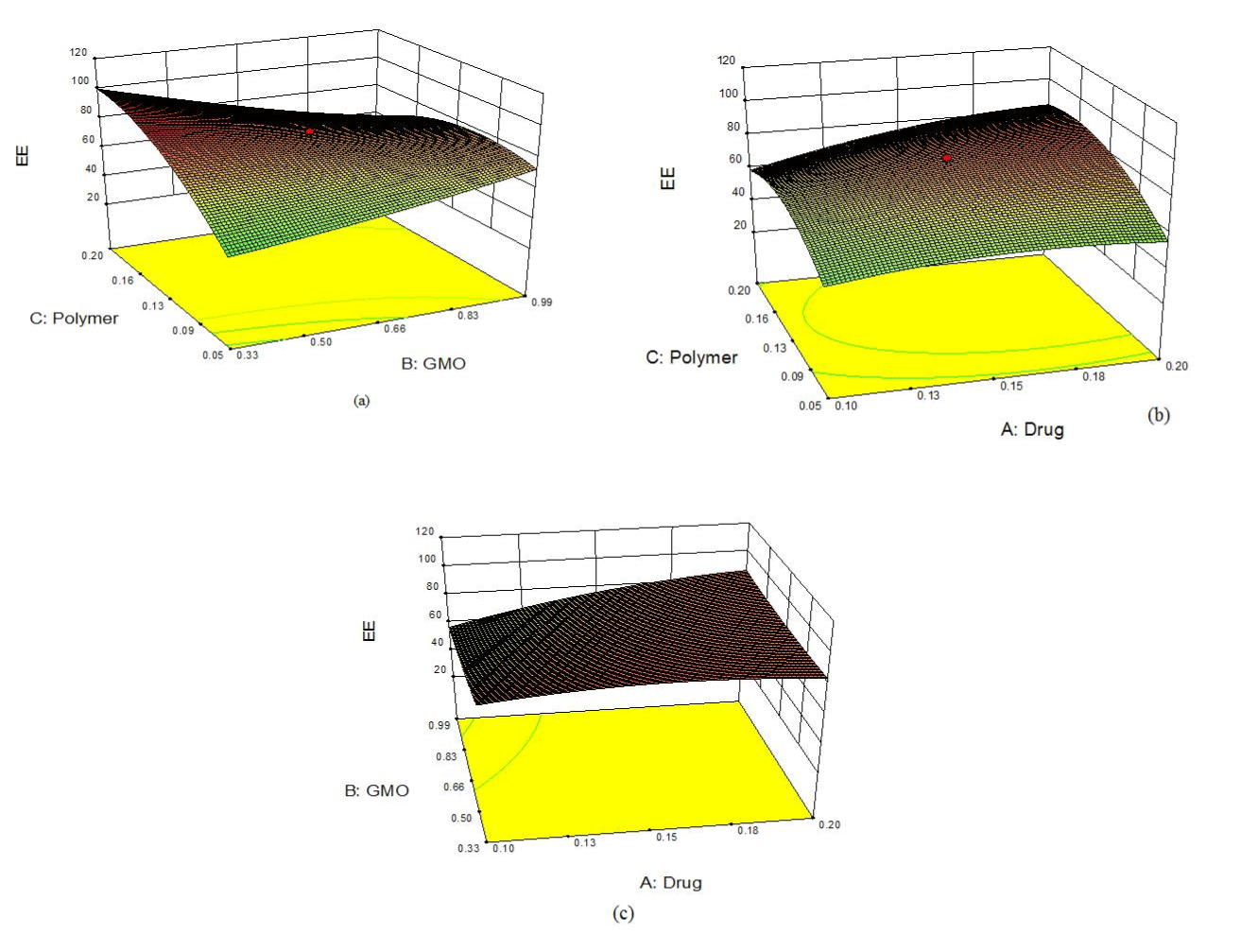
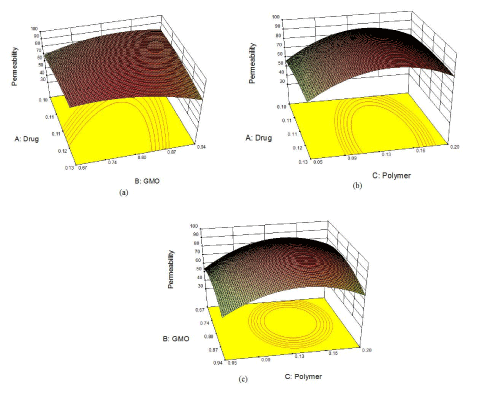
| On applying the numeric optimization method to search for an optimal formulation, four best suggested formulations were selected. The batches were prepared and resulting experimental responses were compared with the predicted responses. The percentage error for each was determined and was found to be between -1.31 and 3.94% (Table 6). Based on these results formulation F4 was selected as optimized formulation. The 3-D plots for the optimized formulation are depicted in Figure 2(a) and 2(b). |
| The optimized formulation was found to offer the best optimal responses in the form of percentage entrapment efficiency and percentage drug permeability. The surface of 3-D plots was slightly convex for entrapment efficiency as well as for percent drug permeability. These shapes suggested that both the responses fitted well in quadratic polynomial equation. Finally, the above data obtained by model analysis indicated the suitability and significance of the selected design, factors, levels and responses. The model graphs clearly show the effects of the factor levels on the responses. It was observed that as the ratio of drug, polymer and lipid increased, there was increase in the entrapment efficiency but the increase is up to a certain extent. The same kind of relationship was also observed permeability at 6th hour. Thus, the 3-D plots of the optimized formulation presented the response surface having the maximum entrapment efficiency along with the permeability, out of the different formulations prepared. |
|
Characterization and evaluation of cubosomes
|
|
Morphological study of optimized cubosomal formulation:
|
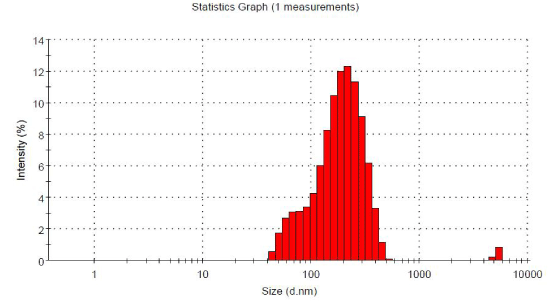
| Transmission electron microscopy (TEM) image of optimized cubosomal formulation is been shown (Figure 3). The results obtained from drug loaded optimized cubosomal formulation showed the morphology of cubic vesicles. The smallest vesicle size observed was 10- 200 nm at magnification of 300000X. The TEM image revealed that the formed vesicles were cubic in nature, thus, confirming the cubosomal formulation. The optimized formulation showed average vesicle size of 171 nm with PI of 0.289 as shown in Figure 4. This shows that the optimized cubosomal formulation was homogeneous with uniform particle size distribution. |
|
Ex-vivo drug permeation studies for cubosomal dispersion:
|
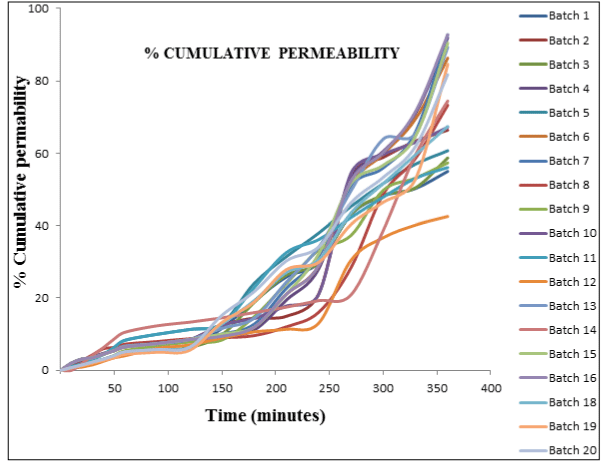
| Ex vivo drug permeation of formulation through goat’s skin at sixth hour was studied which was considered as one of the response in optimization study [28]. From the drug permeation studies, it was observed that formulation F12 that contained minimum quantity of three components (drug:lipid:polymer) offered lowest permeability, whereas with increase in the amount of the components at specific ratio, F19 offered the maximum drug permeability (Figure 5). This study indicated that the quantity and ratio of the components affect the permeability. It was observed that with increase in the quantity of lipid (GMO), the permeability of the drug through the skin also increased up to certain extent. The reason behind the response was composition of lipid, which is a fatty acid and offers similarity with the structure of the skin [11,34]. The lipid contributes to the fluidity of the membrane and modifies the stratum corneum by making it more permeable for drugs. However, the amount of lipid was not the only factor, which affected permeation as observed in formulations F1, F3 and F11 where, maximum amount of lipid was present but still the percentage permeability of drug was between 54-58%. This indicated that the permeability was also dependent upon other components (amount of polymer and drug). The difference in the permeability was seen when the amount of polymer was changed in the formulations. This could happen because of the variation in viscosity of the polymer [10]. As the amount of the polymer increased, dispersion attained gel phase leading to delayed release. Amount of drug available for diffusion also displayed notable effect on the drug permeation. The permeability decreased with increase in the amount of drug above a certain limit. After evaluating all the formulations, it was construed that the batch F4 having composition of the components within optimum range offered (91%) the maximum permeability. From the above study, it can be stated that the ratio of the components affect the permeability. Thus, importance of determining the optimum formulation with required permeability becomes pertinent. |
|
Evaluation of cubosomal gel
|
|
Rheology of gel and texture analysis:
|
| The cubosomal gel was prepared with the composition shown in Table 7. Rheological investigations are basically concerned with determination of the relationship between shear stress, stress rate and viscosity [26]. Power law equation is widely applicable. It is given as: |
| γ = Ktn (3) |
| Taking logarithm on both the sides |
| Log γ = Log K +n Log t (4) |
| Where, τ is shear stress, γ is shear rate, K is consistency index and n is flow index. |
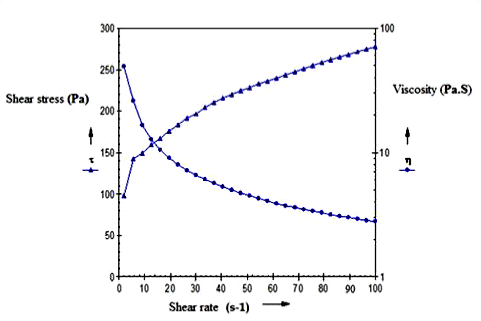
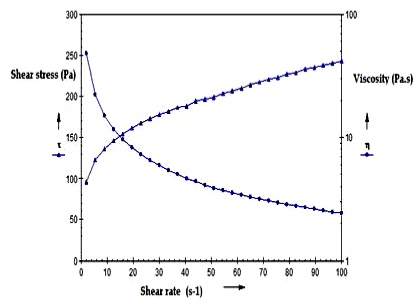
| The viscosity of the optimized formulation and marketed formulation was determined at 37°C with cup and bob rheometer using approximate 15 g of sample (Figures 6 and 7). The plot of shear stress Vs shear strain was obtained. It was observed that both the gels followed pseudoplastic behaviour along with the viscosities which were equivalent for both the marketed as well as optimized cubosomal gel. |
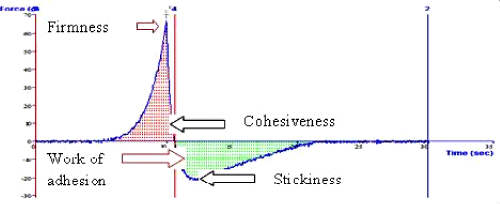
| The prepared gel and marketed gel were analysed for various parameters of texture such as firmness, consistency, stickiness and work of adhesion (Table 8). All the above mentioned properties are important to provide bioadhesiveness and proper spreading of the gel to provide effective action. Cubosomal gel presented slightly higher consistency and work of adhesion as compared to marketed gel. It was clear from the above results that there was negligible difference in various texture characteristics of optimized cubosomal gel and marketed gel of fluconazole (Figures 8 and 9). |
|
Ex vivo permeation study for cubosomal gel:
|
| In order to compare ex-vivo permeation of cubosomal gel and plain gel, enhancement ratio was also computed as shown in Table 9 respectively. Flux obtained by optimized cubosomal formulation was 1.6 folds higher than the plain gel indicating the enhanced efficiency of cubosomal gel to improve the permeability of fluconazole into the dermal region as compared to the conventional gel. The increase in the permeability of the cubosomal gel could be because of the strong bioadhesive nature of the cubic vesicles. Moreover, the drug loading of the cubosomal gel was higher due to better drug entrapment than the conventional gel [35]. The concentration gradient between the polymeric matrix and medium was more which offered increased flux than the marketed gel. The permeability of the cubosomal gel with high pay load can help the drug to reach and show its effect at the site of action. |
| Different mathematical models were used to understand the release mechanism of cubosomal formulation. Table 10 shows the r2 and k values of the model equation. The model with r2 value nearest to 1.000 was considered as the ‘best-fit’model for the formulation. The maximum n values were found to be for kosmeyer peppas model and r2 values for zero order model, this shows that the release kinetics follows kosmeyer peppas model, the formulation with n>1 indicated release as super case II transport [29]. In super case II transport, initially the release remains linear with time and it depends upon the solvent concentration for diffusion, but after some time the rate suddenly gets increased [36]. The reason behind the sudden increase in the diffusion coefficient as compared to the concentration can be solvent retention in the matrix [37]. The release of the solvent with the drug then follows non-linear kinetics. Furthermore, high r2 values for zero order model showed that the release rate was independent of the concentration of the drug dissolved. |
|
In vitro antifungal study
|
| The antifungal activity of fluconazole from optimized cubosomal gel was compared with Flucose® gel (Figure 10). The activity was determined by measuring the zone of inhibition [38]. The optimized cubosomal formulation exhibited good zone of inhibition above the range of minimum inhibitory concentration (MIC 25-50 μg). It was observed that the inhibitory effect was more pronounced for the cubosomal gel with the average inhibition zone of 35 mm whereas; Flucose® gel offered the average inhibition zone of 32 mm [30]. This indicated good correlation between the chosen formulation and the results of in vitro antimicrobial susceptibility testing. From the study, it was observed that the antimicrobial activity can be enhanced due to higher drug loading and better diffusion of the optimized cubosomal gel. Thus, the effectiveness of the treatment may be improved that in turn, would improve the patient compliance. |
Conclusions
|
| In the present study, GMO based cubosomal gels of fluconazole were prepared using different components in different combinations. The final formulation was optimized by applying experimental design technique. It was observed that the novel cubic vesicles were able to enhance the drug payload, offered good entrapment efficiency and enhanced drug permeability as compared to the conventional gel of fluconazole. The study altogether indicated that fluconazole loaded cubosomal gel can serve as a potential topical antifungal gel to treat the fungal infections like candidiasis and leishmaniasis. |
Acknowledgements
|
| The authors wish to acknowledge the funding provided by P.S.C.S.T (Punjab State Council for Science and Technology) for this project. The authors are grateful to Symed Lab Ltd. (SREC arcade, Andhra Pradesh, India) for providing free gift sample of fluconazole and Danisco Pvt. Ltd, (Denmark) to provide free gift sample of the lipid, Glyceryl mono oleate (GMO). |
Declaration of Interest
|
| The authors hereby declare no conflict of interest. |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
 |
 |
 |
 |
 |
| Table 6 |
Table 7 |
Table 8 |
Table 9 |
Table 10 |
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2a |
Figure 2b |
Figure 3 |
 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
Figure 7 |
 |
 |
 |
| Figure 8 |
Figure 9 |
Figure 10 |
|

















