Keywords
Glutathione; Hydrogel; Ocular delivery; Rheology; Newtonian behavior; Sustained release; Permeation
Introduction
Glutathione (γ-glutamyl-cysteinyl-glycine; GSH) is a highly hydrophilic thiol tri-peptide with a low-molecular weight of 307.4 Da [1,2]. The reduced form of GSH serves as a strong intracellular antioxidant and plays an important role in the protection of the eye from oxidative stress [1,3,4]. In addition to its antioxidant activity, GSH serves as a cofactor of enzymes involved in the degradation of peroxides, such as GSH peroxidises. Moreover, as a component of NADPH pathway, it prevents cell components from being oxidized thus promoting DNA regulation and protein synthesis [3]. Franco et al. also reported the role of GSH in the regulation of cell-cycle, signal transduction and immunoresponse [5]. Given that all ocular tissues contain GSH as a primary antioxidant, replenishing the amounts of GSH in the eye could reduce the incidence of ocular diseases related to oxidative stress and aging, such as cataracts. The cornea is particularly susceptible to chemical insults and damage because it is the outer-most layer of the eye and therefore exposed to the harsh environment. GSH maintains normal hydration, protects the cellular membrane and degrades xenobiotic agents in the cornea [4]. Therefore, GSH may play a role in the treatment of keratitis and other corneal diseases [4].
Due to anatomical and physiological characteristics, the eye is uniquely shielded and a highly protected organ that presents many barriers to effective ocular drug delivery [6]. The main physical barriers shielding the eye are tear film and cornea, which protect the anterior of the eye, Tear film is a protective layer that prevents the entry of foreign molecules into the eye. The blood-retina barrier, which protects the back of the eye [7]. The cornea is the main barrier against drug penetration to the inner tissues of the eye due to its small surface area and relative impermeability [8]. In addition, many ocular enzymes in the cornea could degrade GSH and prevent its therapeutic effect. These enzymes include endopeptidases (plasmin, collagenase) and exopeptidases, such as the hydrolytic aminopeptidase, which degrade amino acids [9].
The main advantages of topical administration are its ease and convenience of use and localized drug effects, thus, reducing systemic absorption and avoiding enzymatic degradation through hepatic first pass metabolism [10]. However, the natural ocular defense mechanisms lead to poor drug bioavailability. Blinking increases the production of the tear film’s aqueous layer which in turn increases tear turnover to approximately 1 μL/min, which is equivalent to 16% of the tear film turnover [11]. Both the removal of excess fluid and tear turnover reduce the extent of drug absorption. Conventional eye drops require frequent administration, with each drop being more than 30 μl, which is estimated to be the maximum volume the eye can accommodate without overflow. Drainage of instilled solution occurs within 15- 30 seconds after administration [12], therefore the majority of the drugs administered are removed before absorption. Although ocular ointments have the viscosity to increase pre-ocular drug retention time, they often cause irritability to the eye, resulting in lower compliance. In addition, their oily medium is not compatible with water soluble drugs such as GSH. Consequently, an ocular formulation with desirable characteristics that shows promising potential would be required to effectively deliver GSH. This delivery system should prevent enzymatic degradation of GSH, enhance its pre-corneal retention and penetration.
Hydrogels can be formed by dispersing polymers in an aqueous medium where they undergo swelling to produce a viscous gel capable of increasing pre-corneal retention time, prolonging contact time by increasing mucoadhesion and controlling drug release [12]. These properties increase trans-corneal absorption and hence the amount of drug reaching the anterior chamber of the eye. Poloxamers are nonionic triblock copolymers comprised of ethylene oxide and propylene oxide, which are non-irritating to the ocular surface [13]. With favorable characteristics such as strong hydrogen-bonding, high Mw and sufficient flexibility to interact with the mucus network contribute to excellent mucoadhesion. Another defining characteristic include their ability to increased stability of drugs, Carbopol is a synthetic high Mw polymer comprised of acrylic acid that are cross-linked with either allyl ether of pentaerythritol or allyl sucrose [3]. They are anionic and therefore offer increased mucoadhesion hence contact time [14]. In addition, carbopol is reported to open the cellular tight junctions and could promote trans-corneal penetration of GSH [15]. PVA has been used extensively in ocular formulations due to its ability to maintain ocular osmotic pressure while PAA has been widely used as a viscosity enhancer [2]. The degrees of polymerization and hydrolysis affect the water solubility of PVA. PVA grades with high degrees of hydrolysis have lower water solubility. In addition, PVA grades containing high degrees of hydrolysis are more difficult to crystallize, due to the presence of acetate groups affects the ability of PVA to crystallize. PAA is a polyelectrolyte which is water soluble at neutral pH, due to the ionization of the pendent carboxyl side chains. It absorbs water, become hydrated and swell. Its hydrophilic property and cross-linked structure make it a viscosity enhancer in drug delivery systems. Therefore, different grades of PAA have the different hydrating and swelling characteristics, and influence the ability to enhance the viscosity of the delivery system [16,17].
In this project, we developed a novel hydrogel formulation which composed of carbopol, PAA and PVA, and used as a carrier system for ocular delivery of GSH. This delivery system is aimed to enhance the permeation of the GSH over the cornea, thus to maximize the bioavailability of GSH via ocular administration.
Materials and Methods
L-glutathione reduced (purity ≥ 99%, Mw 307.32, water solubility: 292.5 mg/ml) was purchased from Sigma-Aldrich (USA). P407 and P188 were purchased from BASF (Germany). C934NF were from Noveon (USA) while C940NF and C1342NF polymer were purchased from Lubrizol (USA). PVA (Mw 85,000-124,000, 99% hydrolyzed) came from Applichem (Germany) and PAA (Mw ~ 2,000) was from Polyscience Inc (USA). Sodium hydroxide (NaOH) was from Scharlau (Spain). All other chemicals and solvents were of analytical grade. Bovine eyes were obtained from Auckland Meat Processors (New Zealand) and stored at –20°C until required.
Materials
Preparation of formulations: The carbopol formulations were prepared by adding the required amount of carbopol to 35 ml of milli-Q water and were continuously stirred until the carbopol was completely dissolved. The solution was cooled to 4°C and the required amount of Poloxamer 407 (P407) and Poloxamer 188 (P188) were added with gentle stirring. The formulation was stored in the refrigerator and stirred every 30 minutes until the Poloxamers were completely dissolved. Milli-Q water was added to make up to a final volume of 50 ml. The pH of the formulations was then adjusted to 7.4 by the addition of a small amount of 1 M of NaOH. Table 1 lists the samples prepared using the method mentioned above.
| Formulation |
P407
Concentration (w/v) |
P188
Concentration (w/v) |
Carbopol
Concentration (w/v) |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.3% |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.5% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.3% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.5% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.3% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.5% |
Table 1: Composition of carbopol formulations without glutathione.
PVA formulations (Table 2) were prepared by adding the required amount (w/v) to 35 mL of milli-Q water, which was then heated to 50°C under constant stirring until all PVA was dissolved and made up to 50 mL with milli-Q water. The pH was adjusted to 7.4 by adding a small amount of 5 M NaOH. The corresponding concentration of PAA samples were added according to Table 2 and dilutions were made with milli-Q water. The control used in the screening of the formulations was milli-Q water [3,16,17].
| Polymers |
Concentration (w/v) |
| PVA |
2% |
| PVA |
4% |
| PAA |
12.5% |
| PAA |
25% |
Table 2: Composition of individual polymer formulations without glutathione.
The polymer combinations were shown in Table 3. The carbopol formulations containing GSH were prepared by dissolving 0.5 g of GSH in milli-Q water. Once GSH was dissolved, the required quantity of carbopol was added. The solutions were continuously stirred until carbopol was completely dispersed and the Poloxamers were prepared according to the method described above. The control for samples mentioned in Table 3 was aqueous solution of GSH. Simulated tear fluid (STF) was prepared based on the method used by Hagerstrom et al. [18]. Based on the physical parameters obtained, several potential formulations with GSH were investigated in this study.
| Formulation |
P407 Concentration (w/v) |
P188 Concentration (w/v) |
Carbopol Concentration (w/v) |
Drug Concentration (w/v) |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
1% |
| C934NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
1% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
1% |
| C940NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
1% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.1% |
1% |
| C1342NF+P407/P188 |
21% (10.5 g) |
5% (2.5 g) |
0.2% |
1% |
Table 3: Composition of formulations with glutathione (1% w/v) incorporated.
Characterization
Rheological properties: The rheological properties were examined using the Brookfield DV-III+rheometer (Brookfield Engineering Laboratories, Inc. USA) with spindle 40 for PVA and PAA samples and spindle 52 for the carbopol formulations. This was carried out in a temperature-controlled environment at 25 ± 1°C to simulate room conditions. Each sample went through 20-40 loops of shearing, in between which there was a 10 second delay. The speed of shearing increased by 5 rpm at each loop and the torque was kept between 9 to 90% by manipulating the starting rpm to ensure accuracy of data. The range of rotational speed was between 1 and 394 rpm corresponding to a shear rate range between 2 and 788 s-1.
Mechanical and mucoadhesive properties: TA-XT plus texture analyzer (Stable Micro Systems, UK) with a 5 kg load cell at 25 ± 1°C was used. Texture analyzer was calibrated. For the measurement of the mechanical properties, a 10 mm diameter delrin cylinder probe was twice compressed into each formulation at the rate of 2 mms-1 to a depth of 5 mm with a trigger force of 0.1 g. A delay of 15 seconds was allowed between the compressions. Data collection and calculations were gathered from the Texture Exponent 3.0.5.0. The force-time plot measured mechanical properties such as hardness, compressibility, adhesiveness and cohesiveness. This experiment was conducted in triplicate.
Hardness is measured by the peak force of the first compression cycle. Compressibility is measured as the positive force area during the first compression of the probe [3,19]. Adhesiveness is the negative force area of the first compression [19]. Cohesive force of each sample is the ratio of the first and second positive force area of the two consecutive compressions.
Mucoadhesive force measurements were performed on freshly prepared bovine cornea using TA-XTplus texture analyzer (Stable Micro Systems, UK) with a 5 kg load cell at 25 ± 1°C. The cornea was stabilised on the mucoadhesion test rig. A small amount of sample was applied to the 10 mm delrin cylinder probe surface, after which the probe was lowered at a constant speed of 2 mms-1 and a trigger force of 5 g. After 10 seconds of contact, the probe was moved away at a speed of 0.5 mms-1, generating a curve. The area under the curve (AUC) was calculated by the Texture Exponent 3.0.5.0 program. The work of mucoadhesion was calculated through Equation 1 [3]. Each measurement was conducted in triplicates.
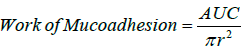 (1)
(1)
where πr² is the surface area of the cornea.
Permeability studies: Ex-vivo drug permeation studies were performed by Franz diffusion cell (VTC 200, Logan Instrument Corp). The thermostat of the Franz diffusion cells was set at 32 ± 1°C to mimic the ocular surface temperature. The Bovine cornea membrane from eye ball was carefully removed using a scalpel (size 20), ensuring good integrity and rinsed with simulated tear fluid (STF) prior to be used. The prepared Bovine cornea with thickness of approximately 0.2 mm is placed between the donor and the receptor chamber. Twelve mL of STF at pH 7.4 served as the dissolution medium in the receptor chamber which functioned as the reservoir. One mL of formulation with 1% w/v drug concentration was placed in the donor chamber and the cap was covered with parafilm to prevent evaporation. Samples (of 0.4 ml) were collected at predeterminate time intervals and replaced by 0.4 ml of STF for the first four hours, after which a greater amount of sample was withdrawn and replaced in order to maintain sink condition. UV spectrometer was blanked with STF at the wavelength of 215 nm and the absorbance of each sample was obtained. The absorbance for samples presented in Table 3 was measured and concentrations were calculated. In order to evaluate the penetration rate, the apparent permeability coefficient (Papp) was calculated, using the following equations:
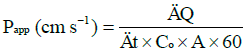 (2)
(2)
Flux 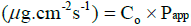 (3)
(3)
where ΔQ / Δt is the steady-state of the linear portion of the graph which presents the amount of drug in the receptor chamber versus time, A represents corneal area available for diffusion (1.766 cm2), Co is the initial GSH concentration in the donor chamber and 60 is the conversion factor from minute to second [2,20]. The linear branch of the permeation data was determined using correlation analysis. A minimum of six data points in the linear branch were taken to calculate the flux, J, (μg.cm-2s-1) by linear regression. The flux was then divided by the concentration in the donor (μg.cm-3) in order to calculate Papp (cm.s-1) [2,21].
Cryo-Scanning Electron Microscopy (Cryo-SEM): Cryo-SEM was used to evaluate the hydrogel structure at swollen state. Images were obtained through Philips XL30S FEG (Field Emission Gun) SEM (Netherlands). Cryo unit, Gattan Alto 2500 was employed, including a fracture stage and a sputter coater, with a coating temperature of less than -120°C. Samples were placed on a brass specimen holder and heated to allow thermogellation. The sample were frozen by using liquid nitrogen (-200°C) and then cut and allowed to heat to -90°C under vacuum. This allowed the frozen water in between gel pores to evaporate, generating the clear structure of polymer hydrogel. The surface of the sample was sputtered with gold for 4 minutes at -120°C, to minimize any charge builds up, after which the samples were viewed under the cryo-SEM.
Statistical analysis
Statistical data was analyzed using Microsoft Excel 2007, with two-way variance analysis (two-way ANOVA) and pair wise comparisons were performed using t-tests. p<0.05 indicates a significant difference.
Results
Characterization of rheological properties
Hydrogel ocular formulations should ideally have a viscosity of around 1000-5000 cP in order to maximize pre-ocular retention time and the delivery of GSH. Formulations with similar viscosities were plotted together for clear and apparent analysis. The 0.3% C1342 formulation was not included as it was too viscous and the method that was used to evaluate rheological properties was not suitable [22]. All carbopol dispersions exhibited non-Newtonian shear-thinning (pseudoplastic) behavior (Figure 1), i.e., decreasing viscosity with increasing shear rate.
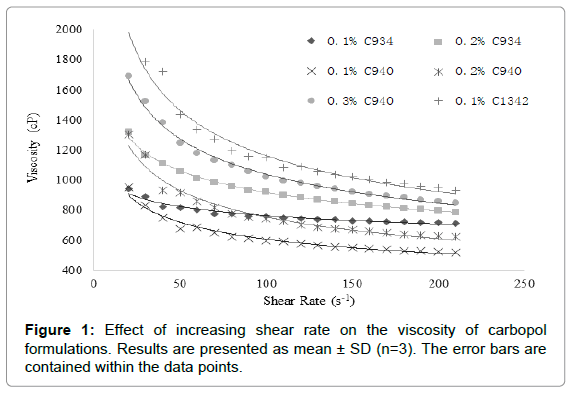
Figure 1: Effect of increasing shear rate on the viscosity of carbopol formulations. Results are presented as mean ± SD (n=3). The error bars are contained within the data points.
It was observed that the increase in viscosity with increasing carbopol concentration was proportionally similar at 0.1% and 0.2% for C934 and C940 at a shear rate of 40 s-1. Conversely, C934 exhibited a much greater increase in viscosity at 0.3% compared to that of C940 (Figure 2). The viscosity difference appears to be proportional between 0.1% and 0.2% of C1342 and 0.2% and 0.3% C934 at the shear rate of 40 s-1. Moreover, the 0.1% and 0.2% C940-containing formulations were the least viscous, while the respective C934 formulations appeared to be 3-fold more viscous than C940-containing formulations (Figure 2). The difference in viscosity between all formulations at different concentrations appears to be significant apart from that between 0.1% C934 and 0.2% C940 and between 0.1% C1342 and 0.3% C940.
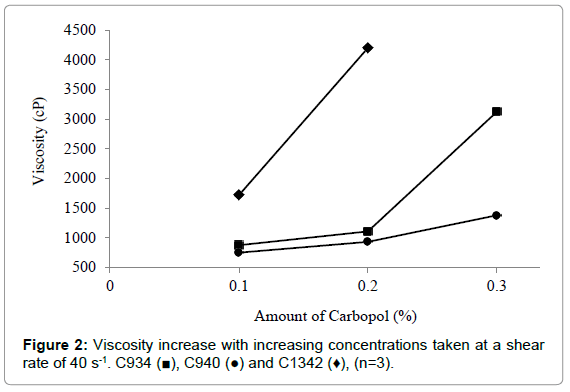
Figure 2: Viscosity increase with increasing concentrations taken at a shear rate of 40 s-1. C934 (?), C940 (?) and C1342 (♦), (n=3).
The difference in viscosity at different concentrations for each carbopol was statistically significant (p<0.05) (Figures 3A-3C). The C940 exhibited the most proportional increase in viscosity with increasing concentration (Figure 3B); while the viscosities of C934 and C1342 appeared to have been more dramatically affected by the change in concentration.
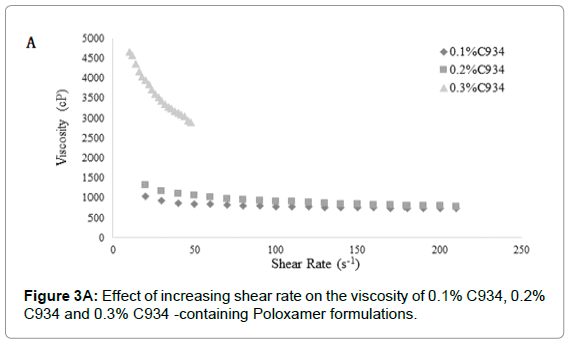
Figure 3A: Effect of increasing shear rate on the viscosity of 0.1% C934, 0.2% C934 and 0.3% C934 -containing Poloxamer formulations.
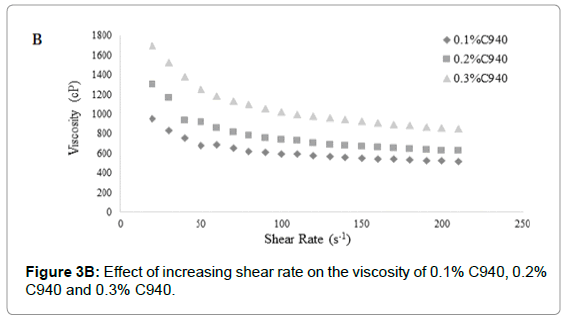
Figure 3B: Effect of increasing shear rate on the viscosity of 0.1% C940, 0.2% C940 and 0.3% C940.
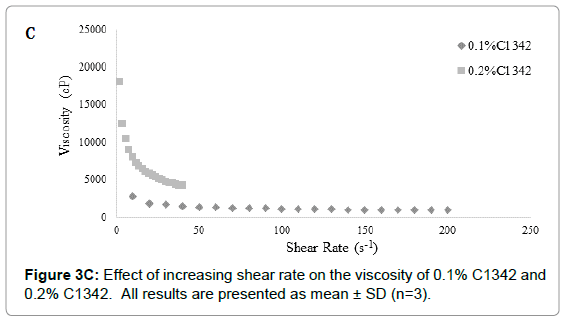
Figure 3C: Effect of increasing shear rate on the viscosity of 0.1% C1342 and 0.2% C1342. All results are presented as mean ± SD (n=3).
The rheological characteristics of PVA and PAA formulations are shown in Figure 4. PVA and PAA results were analyzed separately from that of the carbopol formulations because their viscosity range was vastly different. The PVA and PAA formulations demonstrated Newtonian flow, where a linear relationship between shear rate and shear stress was evident (Figure 4) [7]. Furthermore, the Newtonian flow properties showed that the viscosity of these formulations remained constant despite increasing shear rate. The 25% PAA is approximately 8-fold more viscous than the diluted 12.5% PAA and has a much higher shear stress than all the other three formulations.
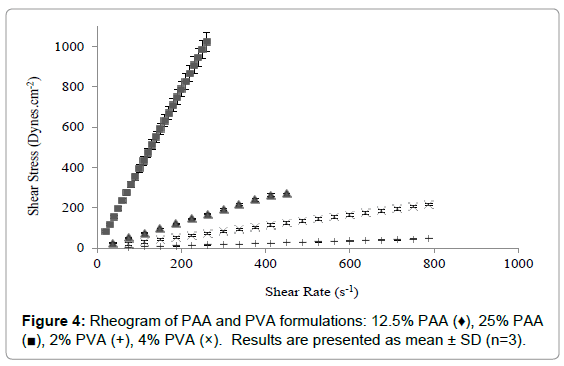
Figure 4: Rheogram of PAA and PVA formulations: 12.5% PAA (♦), 25% PAA (?), 2% PVA (+), 4% PVA (×). Results are presented as mean ± SD (n=3).
Similar to the carbopol systems, the viscosity of PAA and PVA also increased with increasing concentration. There was a significant difference between the viscosities of these simple chain polymers where PAA appears to be more viscous than PVA. The 25% PAA displays a viscosity which is comparable to 0.1% C940-Poloxamer formulation after shearing (Figure 2).
Based on the optimal ocular formulation characteristic described by existing literatures available, the formulation demonstrating the most desirable rheological properties contains the 0.2% C940, it exhibited the lowest viscosity at the lowest shear rate investigated.
Characterization of mechanical and mucoadhesive properties
The hardness and compressibility of the hydrogel system during formulation screening increased with an increase in carbopol concentration. This trend can be observed for all carbopol-containing formulations (Figures 5A and 5B). Significant increase in hardness for C940 and C1342-containing systems was observed at concentration of 0.2% compared to concentration of 0.1% and there was significant difference compared to control (p<0.05). However, no significant differences between the carbopol systems and control at 0.1% (p>0.05) were observed. At 0.3% and 0.5%, all carbopol systems were significantly different, compared to control (p<0.05). Conversely, PVA showed no distinctive change compared to control and no significant differences between the different concentrations was observed (p>0.05). PAA exhibited a significantly lower hardness compared to control (p<0.05), however, there was no difference when concentration was doubled. The hardness of the hydrogel in the presence of PVA was no different when it was compared to all 0.1% carbopol formulations (p>0.05). The results obtained were comparable to the previous literature data [23]. The compressibility of the hydrogel in the presence of C1342 at 0.3% and 0.5%, as well as C940 0.5%, was significantly different from control (p<0.05). Similar to hardness, PAA showed a significantly lower compressibility in comparison to control (p<0.05).
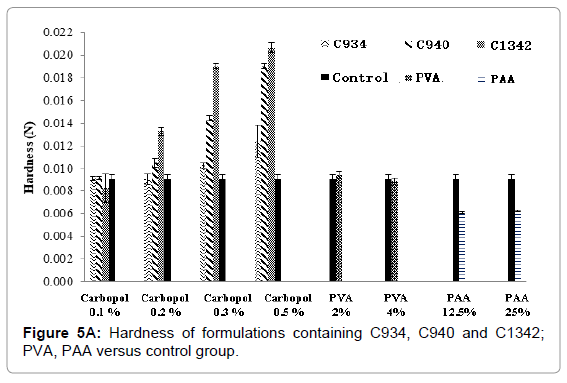
Figure 5A: Hardness of formulations containing C934, C940 and C1342; PVA, PAA versus control group.
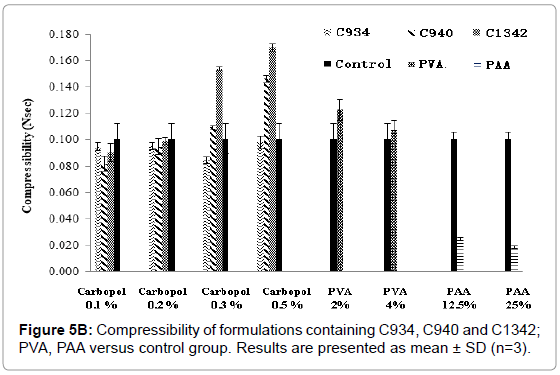
Figure 5B: Compressibility of formulations containing C934, C940 and C1342; PVA, PAA versus control group. Results are presented as mean ± SD (n=3).
A relationship exists between the increase in adhesion and a decrease in cohesion of formulations (Figures 6A and 6B), with a concentration increase. At 0.1%, C1342-containing formulations were significantly higher compared to control (p<0.05). However, adhesion of all carbopol-containing formulations increased with an increase in concentration. Conversely, for PVA and PAA formulation, no such trend can be observed with increase in concentration, and statistical analysis showed no significant difference when compared to control (p>0.05).
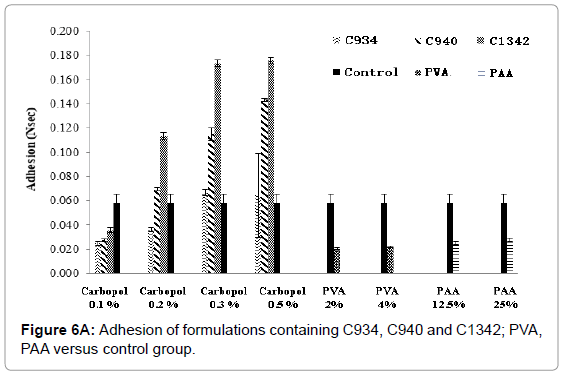
Figure 6A: Adhesion of formulations containing C934, C940 and C1342; PVA, PAA versus control group.
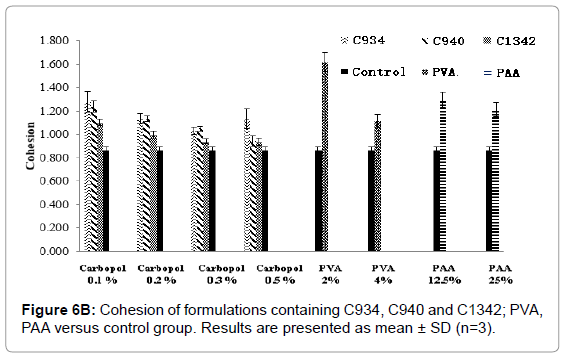
Figure 6B: Cohesion of formulations containing C934, C940 and C1342; PVA, PAA versus control group. Results are presented as mean ± SD (n=3).
At 0.1%, 0.2% and 0.3% of C934- and C940-containing systems, the cohesive forces were significantly higher when compared to control (p<0.05), but this was not demonstrated at a concentration of 0.5% (p>0.05). Cohesion of 0.1% and 0.2% of C1342-containing formulations were significantly higher compared to control (p<0.05). The cohesion forces of 0.1%, 0.2% and 0.3% of C934-containing formulations were significantly different compared to 0.5% of C1342, C940 and 0.1% of C940 respectively (p<0.05). The PVA and PAA formulations demonstrated higher cohesive forces than control (p<0.05) and showed a concentration dependent relationship similar to the carbopol formulations. There was no significant difference in cohesion between PVA and control (p>0.05), but both PAA concentrations had significantly lower cohesion compared to control (p<0.05).
The measured apparent mucoadhesion showed a difference between the control and all formulations screened (Figure 7). It also showed a consistent increase in mucoadhesive force with increase in concentration, however, there was no significant difference between different carbopols at concentrations 0.1% and 0.3%. At 0.2%, only apparent mucoadhesion of C940 was significantly different to that of other carbopols. At 0.5% all carbopol formulations were significantly different from each other. PVA and PAA showed a similar trend, PAA at 25% obtained similar results to 0.5% C934-containing formulation.
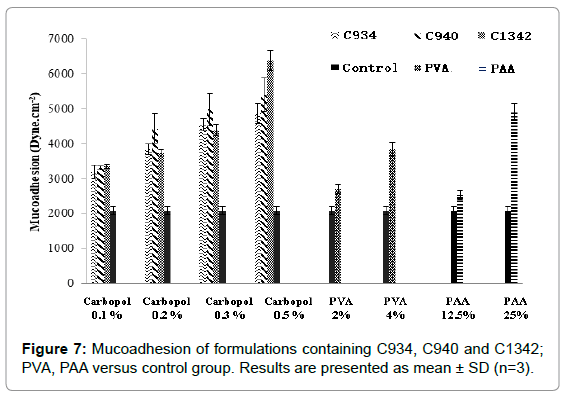
Figure 7: Mucoadhesion of formulations containing C934, C940 and C1342; PVA, PAA versus control group. Results are presented as mean ± SD (n=3).
The cumulative diffusion profiles of each various hydrogel formulations of GSH over eight hours are shown in (Figures 8A- 8C). All of the hydrogel systems showed a linear diffusion trend and showed a significantly higher diffusion when compared to the control (p<0.05). The permeation of 0.1% C940 formulation was significantly less than 0.1% C934, 0.1% and 0.2% C1342 (p<0.05). The remaining formulations did not show any differences when compared to each other. The ranking order of the different formulation groups in their ability to promote GSH diffusion across bovine cornea is: 0.2% C940 and 0.1% C1342>0.1% C934, 0.2% C1342 and 0.2% C934>0.1% C940>control.
Figure 8A: Cumulative amount of GSH as a function of time from various hydrogel systems of 0.1% C934 (?), 0.2% C934 (×) and Control (♦).
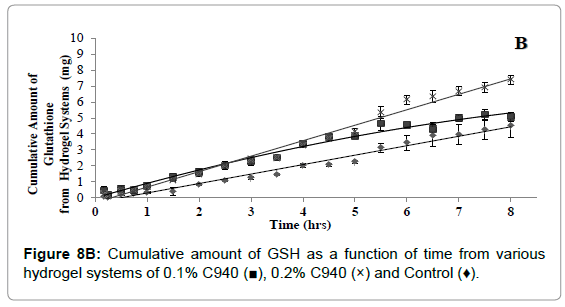
Figure 8B: Cumulative amount of GSH as a function of time from various hydrogel systems of 0.1% C940 (?), 0.2% C940 (×) and Control (♦).
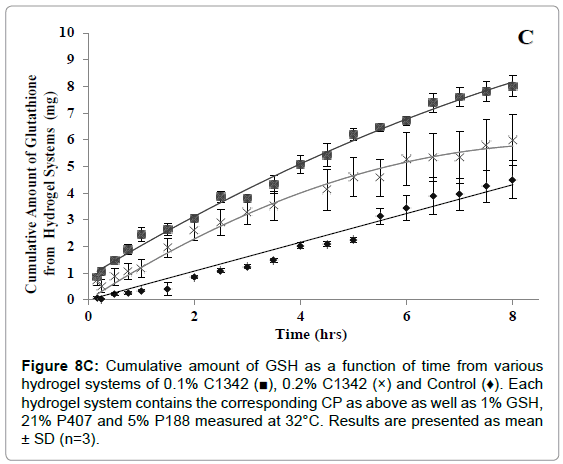
Figure 8C: Cumulative amount of GSH as a function of time from various hydrogel systems of 0.1% C1342 (?), 0.2% C1342 (×) and Control (♦). Each hydrogel system contains the corresponding CP as above as well as 1% GSH, 21% P407 and 5% P188 measured at 32°C. Results are presented as mean ± SD (n=3).
The apparent permeability coefficient (Papp) and Flux values were calculated and shown in Figure 9. From the Papp of the different formulations measured, the permeability of GSH was increased compared to water (p<0.01). The 0.2% C940 (p=0.03) and 0.1% C1342 (p=0.05) had an approximately 1.2-fold higher corneal permeability than 0.1% C940. All other hydrogel formulations did not show differences in their permeability (p>0.05). The average Papp values of the hydrogels were in the range of 1.21-1.57 × 10-4 cm.s-1 compared to 0.645 × 10-4 cm.s-1 produced by the control. This shows that the carbopol combinations enhance the permeability of drug by up to a 2.4-fold (p<0.05).
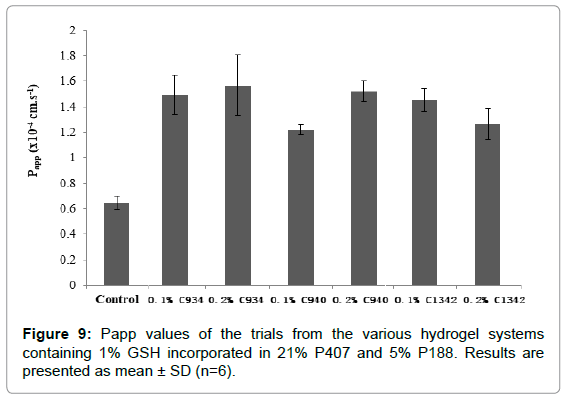
Figure 9: Papp values of the trials from the various hydrogel systems containing 1% GSH incorporated in 21% P407 and 5% P188. Results are presented as mean ± SD (n=6).
The Cryo-SEM images demonstrate the polymeric structure of the achieved carbopol hydrogel systems. Images of C934 and C1342 hydrogel systems with incorporated GSH were obtained (Figure 10). Although, attempt was made to obtain images of C940 hydrogel structure, this could not be achieved due to experimental error. An air bubble was formed during preparation and, as it was cut, no formulation remained on the metallic rib for imaging; thus, the images of C940 hydrogel structure were not obtained and displayed.
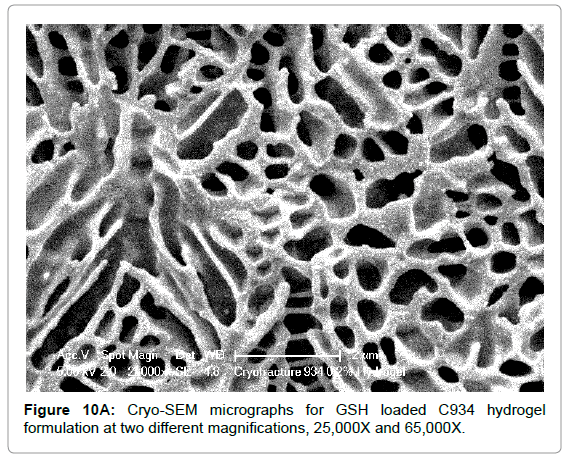
Figure 10A: Cryo-SEM micrographs for GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X and 65,000X.
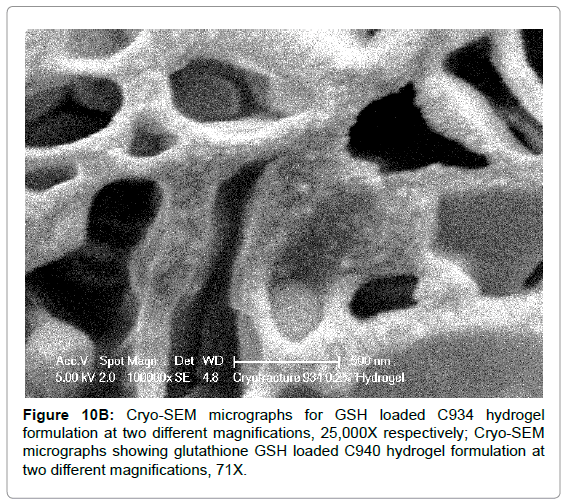
Figure 10B: Cryo-SEM micrographs for GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X respectively; Cryo-SEM micrographs showing glutathione GSH loaded C940 hydrogel formulation at two different magnifications, 71X.
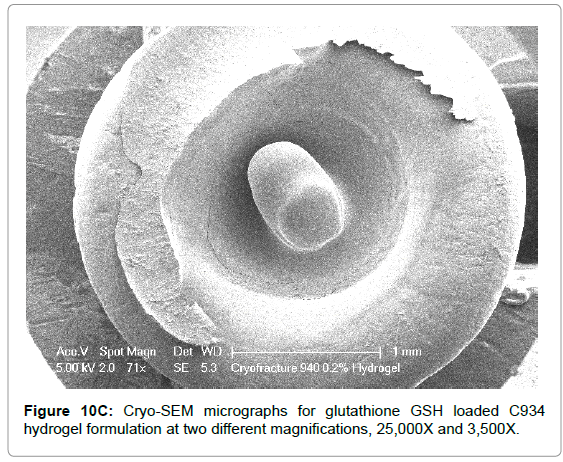
Figure 10C: Cryo-SEM micrographs for glutathione GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X and 3,500X.
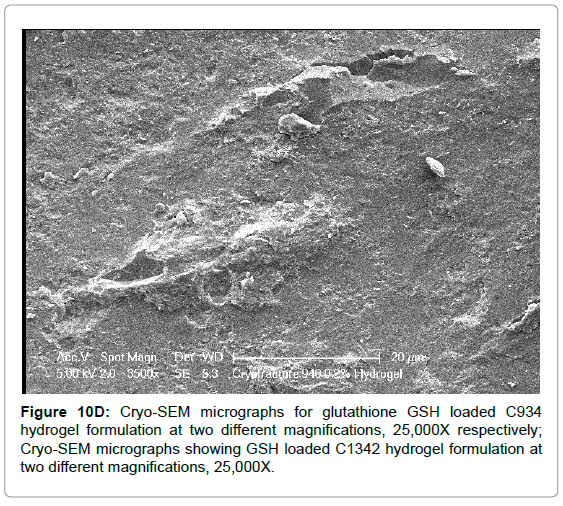
Figure 10D: Cryo-SEM micrographs for glutathione GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X respectively; Cryo-SEM micrographs showing GSH loaded C1342 hydrogel formulation at two different magnifications, 25,000X.
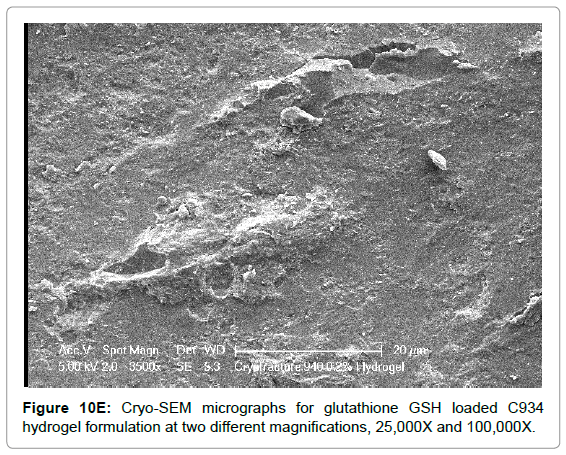
Figure 10E: Cryo-SEM micrographs for glutathione GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X and 100,000X.
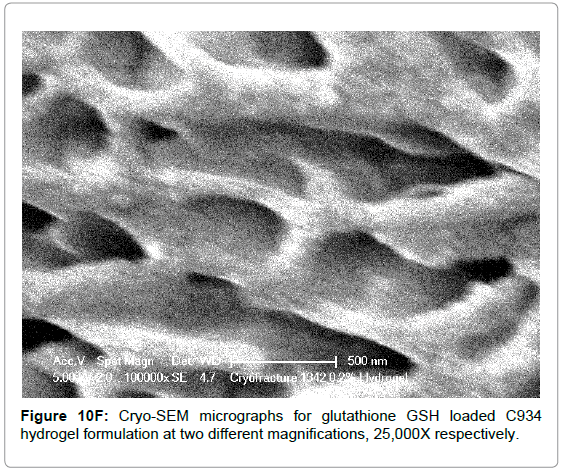
Figure 10F: Cryo-SEM micrographs for glutathione GSH loaded C934 hydrogel formulation at two different magnifications, 25,000X respectively.
Discussion
In order to increase the bioavailability of GSH (and hence achieve post-corneal concentration), the pre-corneal residence time or penetration of the ocular formulation must be extended beyond that of the conventional formulations [24]. This may be achieved by increasing the viscosity, spreadability or mucoadhesion of the formulations; or by incorporating permeation enhancing materials into the formulation. A two-fold increase in maximum bioavailability of a drug was achieved by enhancing the viscosity. Therefore viscosity is often considered as the major factor influencing ocular retention [12]. However, considering the fact that increasing viscosity delays the onset of drug action, thus it will affect the drug absorption over time and bioavailability to some extent. The increase in retention time of instilled formulations is apparent, once viscosity is greater than 10 mPas but a very high viscosity results in ocular discomfort and epithelial damage can occur from blinking [25]. The difference in the effective amount of polymers evaluated was likely the reason for the higher shear stress observed in this experiment. Upon the addition of NaOH solution to adjust pH to 7.4, carboxyl groups on the carbomers (pKa 3-5) are ionized, leading to electrostatic repulsion and dramatically increasing the rigidity of the formulation structure [26]. Thus, the ionized carboxylic groups can interacts with the small but very positive sodium ions, leading to a reduction in viscosity and shear stress. This theory could have been validated, had the rheological characterization been carried out at both pH 5 and pH 7. Although Davies et al. found the viscosity profile of PVA to be pseudoplastic in contrast to our findings; the conditions they used were extreme (85°C) and would not occur in the eye [12]. Moreover, Davies et al. prepared their PVA solution with PBS, introducing ions that possibly change the interactions between the molecules [12]. Nevertheless, C934 was found to have greater viscosity-enhancing capability than PVA. Structurally, PAA are carbopols without cross-link units and can be described as linear polymers (pKa ~ 4.5) [27]. Although PAA has similar rigidity as the carbomers, it is less viscous because the strength provided by cross-links is much more robust.
The structures of hydrogels are highly dependent upon the interaction between the polymers and the swelling medium [28]. The mechanical properties of the hydrogel formulations were assessed through hardness, compressibility, adhesion and cohesion. The ability to prolong ocular contact time was measured through its strength of mucoadhesion [23,29,30]. The hardness is desired to be low in order for the formulation to be easily applied onto the ocular surface [30,31]. Therefore, lower concentrations of carbopol formulations (0.1% and 0.2%) are favoured. The “hardness” of anionic gels is dependent on the molecular network density and the degree of cross-linking, which is correlated to the polymer concentration, as well as viscosity [32- 34]. C1342-containing hydrogels exerted the greater hardness out of all three carbopols. This is likely to be due to an increase in cross-linking between its long alkyl methacrylate chain and allyl ethers of pentaerythritol and its high molecular weight compared to C934 and C940 [35]. Similarly, C934 with relatively lower molecular weight was the easiest formulation to deform due to its reduced rigidity. Additionally, surface molecules have a net inward force due to the cohesive forces being stronger than adhesive forces with the air, therefore a surface energy is created in parallel to the surface. This requires energy to break, which would have been measured as hardness [36-38]. The hardness of PAA was lower than the control. This may be explained through the accumulation of PAA molecules on the surface, leading to reduction in surface tension [36,37]. Compressibility claimed to measure the ease of spread of the formulations over the corneal surface; where a lower value was favorable. With increasing concentration, total resistance was greater hence more force was required as the parameter quantifies deformation under shear and compression [31]. The claimed “cohesion” is described as the spatial reformation of the hydrogel structure after successive compressions. It attempted to measure the relaxation of the polymers, which is dependent on time and the deformation [39]. The “cohesion” of the formulation hoped to examine the ability of the hydrogel to reform its structure after application to the ocular surface, where a high value was desired for maintenance of its structural integrity [30]. A general decline in cohesive force is shown with increase in concentration, which is due to the increase in mass of the polymers [40]. The adhesion is defined to be the interaction between different molecules within the hydrogel. This estimates the ability of the formulation to adhere to the target site [23]. At higher concentrations, there were more polymers present with greater capability to form chemical bonds [22]. The results showed a plateau at concentrations 0.3% and 0.5% for all carbopol-containing formulations. This may be explained by the high cohesive forces within the hydrogel structure to prevent adhesive forces forming with the probe [31]. The degree of mucoadhesion is dependent on the hydration, anionic charges, molecular weight and cross-linking of the polymer [35,41]. All carbopol-containing formulations were combinations of Poloxamers and carbopols. This combination increased its mucoadhesive properties as the polyether components of Poloxamers were able to form tertiary carbon bonds with PAA units of carbopols, which may potentially expose different binding groups to increase mucoadhesive interactions [37,42]. Moreover, the carbopol-containing systems at 0.1% and 0.2% had a greater degree of swelling, therefore increased hydration. The polymers are flexible and free to diffuse to come in close contact with the corneal surface and interpenetrate with the mucus layer, to create a strong entangled network through non-covalent interactions [22,41].
The cumulative amount of GSH that diffused across the cornea from the original administered dose (1 mL), after eight hours ranged from 50.6% to 80.1% from the hydrogels compared to 45.0% from the control system. This is very high compared to the 5% or less, which is typically seen to reach the aqueous humour in living human eyes [43]. This is due to the absence of other ocular barriers such as tear turnover, tear dilution, lacrimation and nasal lacrimal drainage in the Franz diffusion cell apparatus [43]. The GSH control group follows a linear trend across the corneal epithelial membrane and represents zero-order kinetics where diffusion was at a constant rate throughout the experiment. Conversely, the GSH-loaded carbopol-containing hydrogels show a biphasic release profile. The initial phase was attributable to the release of GSH from the outer pores of the gel surface and the second phase showed the sustained release of GSH from the matrix of the polymer. This release profile shows benefits on application, rapid diffusion of GSH through the corneal epithelial membrane will occur, followed by a sustained diffusion over time.
Conclusion
Glutathione is a potent anti-oxidant that is essential in the maintenance of tissue health. By replenishing the tissues with GSH, it is hoped that the progression of ocular damages can be halted or even reversed. Hydrogels composed of carbopol-Poloxamer, PAA and PVA were formulated and investigated as potential delivery systems for GSH across the cornea. The carbopol-Poloxamer combinations exhibited favourable pseudoplastic behavior and cohesion/adhesion properties. These were further investigated for their ability to increase GSH permeability across excised bovine corneas using a Franz diffusion cell. Penetration of GSH across the cornea was significantly increased compared to the plain drug solution. The 0.2% C940- containing-Poloxamer formulation was determined to be a promising drug delivery system for ocular delivery of GSH. To study the efficacy of GSH formulations on the reversibility of cataracts, in vivo tests can be performed in the future.
24304
References
- Chen G (2012) Mucoadhesive film and PLGA based nanoparticle as carrier systems for sublingual delivery of glutathione. p: 276.
- Chen G, Wen J (2018) Poly (lactic-co-glycolic acid) based double emulsion nanoparticle as a carrier system to deliver glutathione sublingually. J Biomed 3: 50-59.
- Chen G, Bunt C, Wen J (2015) Mucoadhesive polymers?based film as a carrier system for sublingual delivery of glutathione. J Pharm Pharmacol 67: 26-34.
- Ganea E, Harding JJ (2006) Glutathione-related enzymes and the eye. Curr Eye Res 31: 1-11.
- Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI (2007) The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem 113: 234-258.
- Gaudana R, Jwala J, Boddu SH, Mitra AK (2009) Recent perspectives in ocular drug delivery. Pharm Res 26: 1197.
- Martin AN (2006) Martin's physical pharmacy and pharmaceutical sciences: Physical chemical and biopharmaceutical principles in the pharmaceutical sciences. Lippincott Williams & Wilkins.
- Davies NM (2000) Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol 27: 558-562.
- Attar M, Shen J, Ling KH, Tang-Liu D (2005) Ophthalmic drug delivery considerations at the cellular level: drug-metabolising enzymes and transporters. Expert Opin Drug Deliv 2: 891-908.
- Chen G, Li D, Jin Y, Zhang W, Teng L (2014) Deformable liposomes by reverse-phase evaporation method for an enhanced skin delivery of (+)-catechin. Drug Dev Ind Pharm 40: 260-265.
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134: 489-492.
- Davies NM, Farr SJ, Hadgraft J, Kellaway IW (1991) Evaluation of mucoadhesive polymers in ocular drug delivery. I. Viscous solutions. Pharm Res 8: 1039-1043.
- Almeida H, Amaral MH, Lobão P, Sousa Lobo JM (2013) Applications of poloxamers in ophthalmic pharmaceutical formulations: An overview. Exp Opin Drug Deliv 10: 1223-1237.
- Asane G, Nirmal SA, Rasal KB, Naik AA, Mahadik MS, et al. (2008) Polymers for mucoadhesive drug delivery system: A current status. Drug Dev Industrial Pharm 34: 1246-1266.
- Hosmani AH, Thorat Y, Kasture P (2006) Carbopol and its pharmaceutical significance: A review. Pharm Rev, p: 4.
- Rudnik E (2013) Compostable Polymer Properties and Packaging Applications. Plastic Films in Food Packaging. Will Andrew Pub, pp: 217-248.
- Ritthidej R, Garnpimol C (2011) Nasal Delivery of Peptides and Proteins with Chitosan and Related Mucoadhesive Polymers. Peptide and Protein Delivery. Academic Press, pp: 47-68.
- Hägerström H, Paulsson M, Edsman K (2000) Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci 9: 301-309.
- Cevher E, Sensoy D, Taha MA, Araman A (2008) Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS Pharm Sci Tech 9: 953-965.
- Muchtar S (1997) Ex-vivo permeation study of indomethacin from a submicron emulsion through albino rabbit cornea. J Controlled Release 44: 55-64.
- Chen G, Svirskis D, Lu W, Ying M, Huang Y, et al. (2018) N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer. J Control Release 277: 142-153.
- Jones DS, Woolfson AD, Brown AF (1997) Textural analysis and flow rheometry of novel, bioadhesive antimicrobial oral gels. Pharm Res 14: 450-457.
- Cevher E, Taha MA, Orlu M, Araman A (2008) Evaluation of mechanical and mucoadhesive properties of clomiphene citrate gel formulations containing carbomers and their thiolated derivatives. Drug Deliv 15: 57-67.
- Asane GS, Nirmal SA, Rasal KB, Naik AA, Mahadik MS, et al. (2008) Polymers for mucoadhesive drug delivery system: A current status. Drug Development and Industrial Pharmacy 34: 1246-1266.
- Zhu H, Chauhan A (2008) Effect of Viscosity on Tear Drainage and Ocular Residence Time. Opto Vis Sci 85: E715.
- Chen G, Svirskis D, Wen J (2015) Development and validation of a stability indicating isocratic HPLC method for gemcitabine with application to drug release from poly lactic?co?glycolic acid nanoparticles and enzymatic degradation studies. J Pharm Pharmacol 67: 528-1536.
- Riley RG, John DS, John TS, Peter WD, Frank H, et al. (2001) An investigation of mucus/polymer rheological synergism using synthesised and characterised poly (acrylic acid). Int J Pharm 217: 87-100.
- Le Bourlais C, Acar L, Zia H, Sado PA, Needham T, et al. (1998) Ophthalmic drug delivery systems-recent advances. Prog Retin Eye Res 17: 33-58.
- Yaprak Karavana S, Guneri P, Ertan G (2009) Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharma Dev Tech 14: 623-631.
- Cevher E, Sensoy D, Taha MA, Araman A (2008) Effect of thiolated polymers to textural and mucoadhesive properties of vaginal gel formulations prepared with polycarbophil and chitosan. AAPS Pharm Sci Tech 9: 953-965.
- Jones DS, Woolfson AD, Brown AF (1997) Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. International Journal of Pharmaceutics 151: 223-233.
- Jones DS, Woolfson AD, Djokic J (1996) Texture profile analysis of bioadhesive polymeric semisolids: Mechanical characterization and investigation of interactions between formulation components. J App Pol Sci, p: 61.
- Tamagawa H, Nogata F, Yagasaki K (2004) An interpretation of amphoteric gel hardness variation through potential and hardness measurement. J Colloid Interface Sci 275: 107-112.
- McTaggart LE, Halbert GW (1993) Assessment of polysaccharide gels as drug delivery vehicles. Inter J Pharm 100: 199-206.
- Blanco-Fuente H, Anguiano-Igea S, Otero-Espinar FG, Blanco-Méndez J (1996) In-vitro bioadhesion of carbopol hydrogels. Int J Pharm 142: 169-174.
- Aulton ME (2007) Aulton's pharmaceutics: The design and manufacture of medicines. Edinburgh; New York: Churchill Livingstone, p: 717.
- Sinko PJ, Martin AN (2006) Martin's physical pharmacy pharmaceutical sciences: Physical chemical principles in the pharmaceutical sciences. Philadelphia: Lippincott Williams & Wilkins, p: 795.
- Schwartz AM, Chatterjee PK, Gupta BS (2002) Chapter II: Surface tension and surface energy. In: Textile Science and Technology. Elsevier, pp: 57-91.
- de Cindio B (1984) Stress relaxation of polymer melts subjected to large uniaxial tension. Polymer 25: 1049-1053.
- Jones DS, Woolfson AD, Djokic J, Coulter WA (1996) Development and mechanical characterization of bioadhesive semi-solid, polymeric systems containing tetracycline for the treatment of periodontal diseases. Pharm Res 13: 1734-1738.
- Ludwig A (2005) The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Deliv Rev 57: 1595-1639.
- Qi H, Chen W, Huang C, Li L, Chen C, et al. (2007) Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm 337: 178-187.
- Gaudana R, Jwala J, Boddu SH, Mitra AK (2009) Recent Perspectives in Ocular Drug Delivery. Pharm Res 26: 1197-1216.







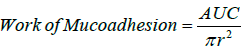 (1)
(1)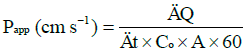 (2)
(2)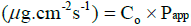 (3)
(3)



















