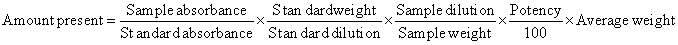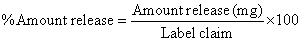Keywords
|
| Colon-targeted delivery; Hard gelatin capsule; Organic acid; Eudragit E 100; CMEC; Capecitabine |
Introduction
|
| In spite the presence of various delivery systems, colon as a site, caters some distinct beneficial features on account of a near neutral pH, a much longer transit time; lessen digestive enzymatic activity and a much greater responsiveness to absorption enhancers [1]. Colon specific drug delivery has been increasingly expanding the researcher’s attention in mammoth for not only in the gastrointestinal segment but also the systemic area of approach as well. Besides, colonic delivery of drugs may be extremely significant when setback in drug absorption is required from a therapeutic view point, e.g., in case of diurnal asthma, angina pectoris and arthritis [2]. Currently available drug delivering methodologies are quite seldom in releasing or targeting the drugs in the upper gastro intestinal tract as for as its absorption and degradation is concern. So a sophisticated alternative stratagem is quite essential to cope up with the aforementioned issues. Ever since the issue, inconsistent massive approaches have been made in the last decade to stabilize a connotation in full move towards the colon region is in existence. In deliberate concern vis-a-vis the physiological conditions of the gastrointestinal tract, various systems have been developed based on different principles, including pH-triggered [3,4], time-controlled [5-8] and microbial controlled deliveries [9-12]. |
| Dually the cause of concern for the colon specific drug delivery outstands in two fronts either large scale reproducibility or the confronting the regulatory constraints before it put forward to the clinicians contentment. The implications in terms of pharmaceutical feasibility concern as for the material selection and pivotal batches using improved practicality. A microbial cleavage strategy utilizing the high enzymatic activity of microflora in the large intestine may be one of the most promising approaches in terms of an excellent site-specific. However, at this juncture, the aforementioned approach does not prove to be handy because the preparation of the systems usually meets the use of new pharmaceutical additives, such as polymers containing azo-bonding and other materials which are microbial degradable in the large intestine. And so only the feasibility of the product reaching the consumer end is quite complicated and perplexed has impelled as many researchers to rejuvenate the therapy module itself in betterment on stood. |
| In spite more practical technologies, possessing contemporary and conventional coating pointing towards drug release are seldom enough to meet the release requirement, as it releases all its contents promptly after gastric emptying time. Subsequently the exploitation of superior pH enteric coating polymer has been in a while, to strive out in delaying the drug release pattern, reason that a miniature fluctuation in pH or ionic condition sounds huge [3,13,14]. The stained-release or timedrelease approach, in which drugs are released on the basis of timecontrolled principle, is also insufficient because the large variation of gastric emptying time in humans makes it difficult to achieve sufficient and reliable colonic availability of a drug by those methods. The most hopeful tool for controlled- release approaches aforementioned illustrations are thought quite practical in terms of huge scale ups, continued improvisation of site selection of drug is as essential as its dosage. |
| Indeed, we contemporarily endeavored to design a new colontargeted delivery system in capsule dimension, which was designed to possess a pH-sensing function and a timed-release function. In this conjuncture, the concept for the design of this novel device and the core notion of drug release are introduced, and the potential for colonic delivery of drugs will be elicited through a cascade of in vitro studies. |
| Capecitabine is a prodrug that is selectively tumour-activated to its cytotoxic moiety, 5-fluorouracil, by thymidine phosphorylase, an enzyme found in higher concentrations in many tumors compared to normal tissues or plasma. This drug enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows down the growth of tumor tissue. Such candidate can be subject to specialized coating and tailoring the microenvironmental pH, by specialized additives to release the considerable amount of drug in time, to the selected target. The selected candidate has got low bioavailability and subsequent clinical inefficacy is always a concern for the clinicians. Having the fact in mind the current method of pH sensing and time dependent release, is a better alternate to improve the bioavailability. |
Design of Dosage Form
|
| When the physical and physiological conditions of the human gastrointestinal tract are considered, a site-specific oral drug delivery seems to be achieved by appropriately integrating a combination of pH-sensing functions and time-controlled release functions into a single pharmaceutical. These two functions of the CTDC could improve the above-mentioned drawbacks of pH-triggered system and time-controlled system. That is, since the gastric emptying time greatly varies depending on various factors, the drug release in the stomach must be minimized. Imparting a pH sensing function to the pharmaceutical should be effective for this purpose. Meanwhile, the passage of pharmaceuticals in the small intestine is known to be less variable, i.e., about 3 ± 1 hr [15]. After gastric emptying, therefore, the time-controlled release function must be effective to deliver the drug to the target site in the intestine. Thus the colon-targeted delivery capsule (CTDC) was designed according to the above-mentioned concept. |
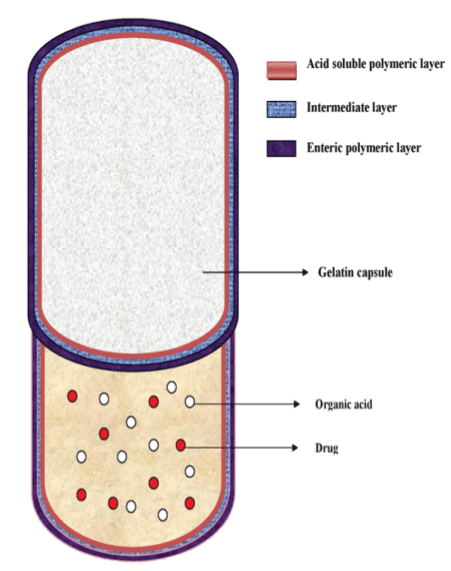
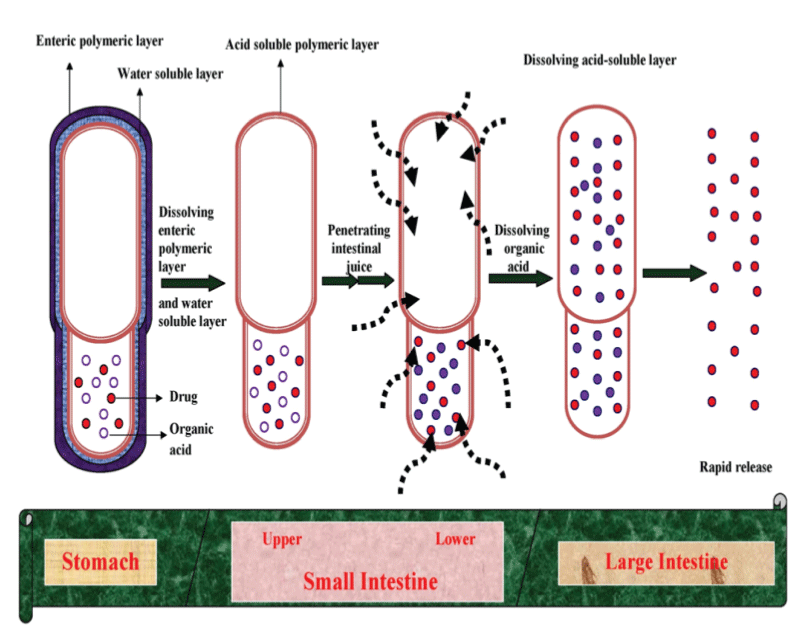
| The fundamental structure of the CTDC is shown in Figure 1. The technical characteristics of this device are to contain an organic acid in a hard gelatin capsule together with an active ingredient, and to coat the capsule with three different polymeric layers; an inner layer consisting of cationic polymer dissolving in acidic fluid, a water-soluble intermediate layer, and an outer layer consisting of enteric materials dissolving at pH>5 [16]. Here, the intermediate layer is provided to prevent the direct contact of the cationic polymeric layer and the anionic polymeric layer. The expected in vivo behavior of CTDC in the gastrointestinal tract is illustrated in Figure 2. After ingestion of the capsule, drug release can be completely prevented in the stomach due to the acid-resistibility of the outer polymeric layer. |
| After gastric emptying, the outer layer and the intermediate layer quickly dissolve, but the inner polymeric layer still remains and effectively prevents the drug release in the intestine. However, when the micro-environmental pH inside the capsule gradually decreases according to the dissolution of organic acid, and when the inner polymeric layer was finally dissolved by the acidic fluid, the drug content was quickly released. The onset of the drug release, therefore, can be controlled by the thickness of the inner polymeric layer. When sufficient acid-resistibility and the suitable thickness of the inner layer are given to adjust the onset time of drug release to 3 ± 1 h, a sitespecific drug release to the proximal colon will be realized. |
Materials and methods
|
|
Materials
|
| Authenticated sample of capecitabine was procured from Naprodifescience Pvt Ltd, Mumbai, India. Hard gelatin capsules (#0, #1, #2, #3 and #4) were purchased from Goutham Pharma Pvt Ltd, Chennai. Eudragit E 100 (Vikram Thermonik Pvt. Ltd. Hyderabad) was used as a cationic polymer which is soluble in a low pH aqueous medium up to pH 5, Carboxy methyl ethyl cellulose (Hetro Lab, Hyderabad) was used as an enteric polymer. Hydroxy propyl methyl cellulose (Chemfield Pharmaceuticals Pvt. Ltd, Mumbai) was used as a neutral water-soluble polymer. Ethyl cellulose (Himedia Laboratories Pvt Ltd. Mumbai) was used as a sealing agent for the hard gelatin capsule. Citric acid, fumaric acid, Maleic acid, Succinic acid and tartaric acid were used as the pH adjusting agents and were purchased from Adlab Pharmaceuticals, Pondicherry. All other chemicals and solvents were of reagent grade. |
|
Preparation of CTDC
|
| Hard gelatin capsules were filled with the powder mixture of Capecitabine, organic acid, etc., were prepared for the experiment purpose. The capsules are subjected for the sealing with Ethyl Cellulose 5% (w/w) in Ethanolic solution, the core capsules obtained were spray-coated with three polymeric films successively in the order of Eudragit E-100, HPMC, and CMEC, using a coating machine (Sakthi instruments Ltd, Pan coater). The formula for the polymeric coating solution and the operating conditions for coating for each layer are described in Table 1. The type of organic acid, the amount of organic acid loaded in a capsule, and the amount of each coating film were changed for optimizing the formulation. In this research, the four trials, namely F1A, F1B, F1C and F1D were carried out to optimize the best formulation. The formulation of CTDC is presented in Table 2. |
|
Measurement of film thickness
|
| Weight of the capsule without and after coating was measured using a electronic balance. The thickness of all the three layers of the capsules after coating was measured using a micrometer screw gauge (Mitutoyo) instrument ranging 0-25 mm with the precision 0.001 mm. Ten different positions were measured for each capsule to obtain a mean thickness and all the results within the limit. |
|
Adjustment of pH inside the CTDC
|
| The pH inside the CTDC was adjusted by filling 100 mg of buffering agents consisting of various ratios of the mixture of succinic acid and borax into the #2 capsule shells. The pH value to be attained in the capsule during the dissolution process was estimated by determining the pH value of the solution dissolving 2.7 g of buffering agent in 10 ml of pH 6.8 (which corresponds to the ratio of the actual volume of the capsule and the content of buffering agent loaded in the capsule). |
|
Drug content and content uniformity
|
| The drug content and uniformity of capecitabine was determined by UV-spectrophotometer. Methanol was used to dissolve the drug for content uniformity assay. The content of 20 capsules was removed and grind into fine powder. Then powder equivalent to 20 mg of capecitabine was accurately weighed and transferred in to 100 ml volumetric flask. About 75 ml of methanol was added and stirred well. This solution was adjusted with methanol to 100 ml. The above solution was used as stock solution and filtered using filter paper. 5 ml was taken from the above stock solution and adjusted to 100 ml with methanol. Then the absorbance was measured at 253 nm and amount of drug present in each capsule was calculated by using the following formula. |
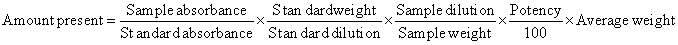 |
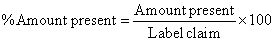 |
|
In vitro release study
|
| The drug release profiles from capsules were investigated according to the procedure described in the Indian Pharmacopoeia (the paddle method). The capsules were placed in a vessel with 900 ml of the pH 1.2 and pH 6.8 at 37 ± 2°C rotating at 100 rpm. The released amount was periodically determined by the spectrophotometric method. All the experiments were carried out in more than triplicate. The morphological change of capsules during dissolution testing in the pH 6.8 was observed using a Digital binocular microscope (Labomed, Netherland). The percentage amount of capecitabine release was calculated by using the following formula |
 |
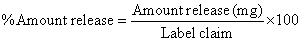 |
Results and Discussion
|
|
Examine of various organic acid on the drug release behavior of drug-loaded CTDC
|
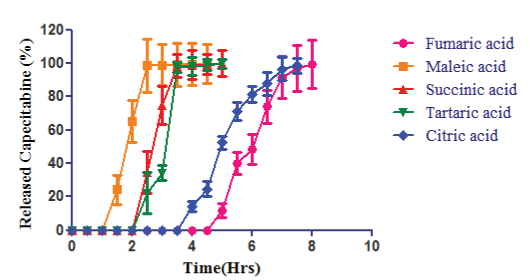
| The release profiles of capecitabine from those capsules in the pH 6.8 are compared in Figure 3. The result shows that the duration of lag time differed depending on organic acid species; for instance, the maleic acid-loaded capsule gave the shortest lag time of about 1.5 h; in the cases of succinic acid and tartaric acid, the lag time observed was about 2.5 h; and in the cases of fumaric acid and citric acid, the lag time was found to be 5 h and 4 h respectively. |
|
Effect of Succinic acid on drug release
|
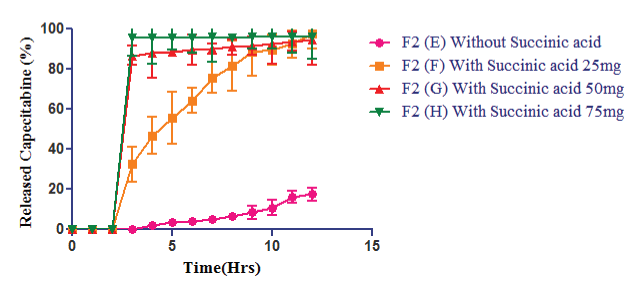
| As was shown in Figure 4, Formulation F2 (E) drug loaded capsule deprived of succinic acid, merely provided a very slow drug release profile of 2.1% at 4th hour and 17.5% at 12th hour respectively. On the other hand, the drug loaded capsules [F2 (F, G and H)] containing succinic acid with different ratio provided a distinct lag time of about 3 hours. That means the drug release profile of [F2 (F, G and H)] are 32.5%, 86.8% and 95.8% and 97.8%, 94.8% and 96.2% at the interval of 3rd hour and 12th hour respectively. |
|
Morphological change of CTDC
|
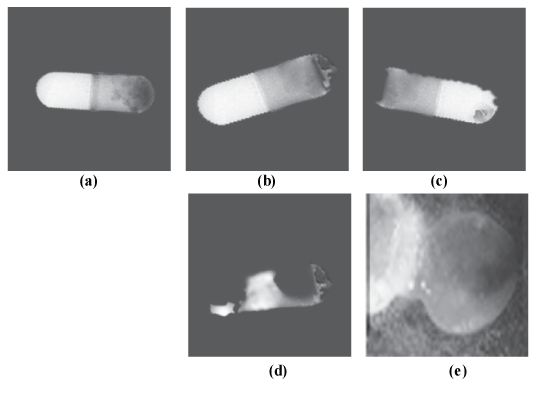
| Figure 5 shows the morphological change of the coated capsules during the drug release process in the pH 6.8. After starting the dissolution test, the outer layer of the capsule (CMEC) and the intermediate layer of the capsule (HPMC) soon dissolved, and the remaining capsule shell with a Eudragit E 100 layer considerably swelled, but no more change in appearance was found within 2.6 h (Figure 5(a)). After 3.1 h, at which about 2% of capecitabine was released, the top portion of the capsule was found to be dented and some holes were observed in it (Figure 5(b)). After 3.5 h, at which about 75% of the drug was released, both ends of the capsule had started to collapse (Figure 5(c)). After 3.9 h, by the time at which all the drug had been released, most of the capsules were dissolved, leaving a small portion of EC film used as a sealant (Figure 5(d)). On the other hand, in the case of the capsule without succinic acid, the CMEC and HPMC layers were dissolved, but the remaining capsule merely swelled and did not dissolve for many hours (Figure 5(e)). |
| Succinic acid-containing capsule: (a) 2.6 h after starting the dissolution test; (b) after 3.1 h (about 2% of capecitabine was released); (c) after 3.5 h (about 75% of capecitabine was released); (d) after 3.9 h (capecitabine release was already completed). Succinic acid-free capsule: (e) 18 h after starting the dissolution test (about 16% of capecitabine was released). |
|
Effect of pH and Lag time relationship of drug release
|
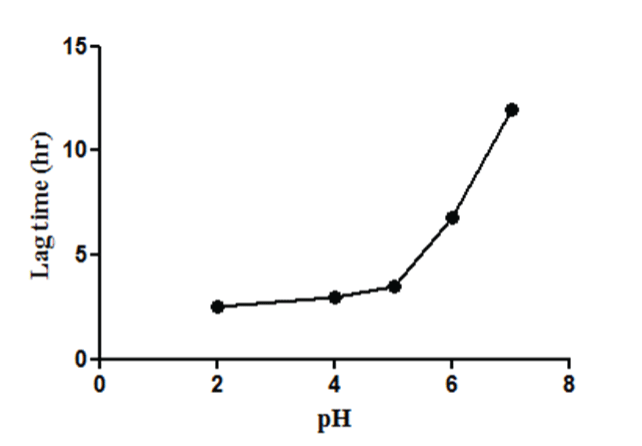
| The relationship between pH inside the capsule and lag time in the pH 6.8 is shown in Figure 6. Above pH 5, the lag time of the Eudragit E 100-coated capsules increased as pH increased, but the drug release rate was slower. Under pH 6.5, however, the lag time was almost stable. The refractive point observed at about pH 6.5 was in accordance with the upper limit of the soluble pH range of Eudragit E 100. |
|
Effect of the loading amount of succinic acid
|
| The observed lag times for preparations are listed in Table 3. As was expected, acid content affected the drug release behavior of the capsules. When the loading amount of succinic acid was less than 50 mg (i.e., 25 mg) per capsule, the lag time was gradually delayed with decreasing the acid content. But, when at 50 mg of succinic acid lag time considerably shortened as compared with the 25 mg of succinic acid. On the other hand, 75 mg of succinic acid load has given a shortened lag time with constant drug release; almost the results of lag time and drug release profiles are comparable and slightly higher, with the 50 mg of succinic acid utilization. |
|
Effect of the coating amount of Eudragit E 100
|
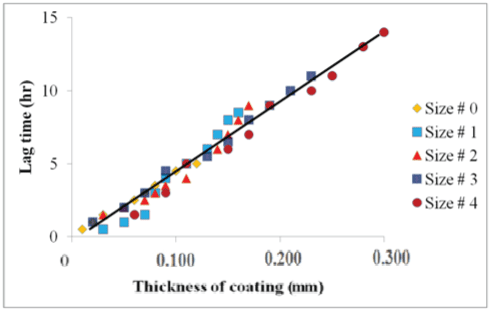
| The lag time observed in the dissolution test for capecitabine loaded capsules in the pH 6.8 was delayed with increased coating, and a very good linear relationship was found between coating amount and lag time (Figure 7). |
|
Enteric protection with CMEC film coating
|
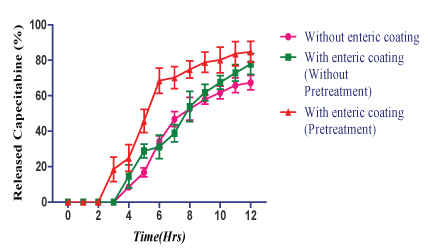
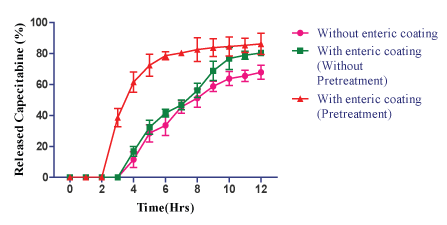
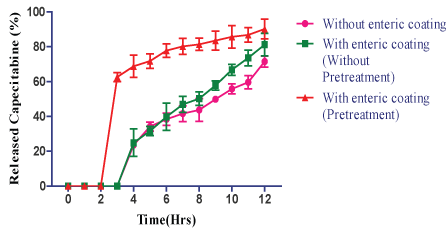
| The results of CMEC coating, the lag time was observed for pH 6.8 was shown that with enteric coating, (without pretreatment) for CEL F2 (E), F2 (F), F2 (G) and F2 (H) released 14.5%, 16.8%, 24.9% and 39.7% at the end of 4th ; 77.9%, 80.4%, 81.2% and 86.8% at 8th h respectively (Figures 8-11). |
| The result shown that with enteric coating (pretreatment by immersing in the 1st fluid for 8 h) in the pH 6.8 for CEL F2 (E), F2 (F), F2 (G) and F2 (H) released 18.5%, 38.6%, 62.6%, 88.6% at the end of 3rd h; and 84.8%, 86.2%, 90.2% and 98.5% of drug at the end of 8th h respectively (Figures 8-11). |
Conclusions
|
| In this context, CTDC can be a useful mean for the colon targeted delivery of the both selected drugs. The effective stratagem of this technology involves: (1) Firstly, various organic acids can be used for this system; (2) Secondly, the predictable timed-release of drug can be attained by adjusting the thickness of inner layer; and (3) Thirdly, the outer enteric coating provided a satisfactory acid resistibility. The CTDC will be able to achieve a higher selectivity to the colon than other time controlled and pH-triggered systems, because it possesses the dual function of both pH-sensing and timing release of drugs. The CTDC has also a surplus advantage from the practical viewpoint, the reason that all the material used is identified as safe in actual usage and the fabrication techniques employed. |
Tables at a glance
|
|
|
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|