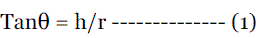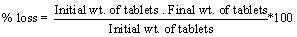Key words
|
| |
| Colon; amoebiasis; ornidazole; physicochemical parameters |
| |
INTRODUCTION
|
| |
| Protozoal infections are more common among the people in under developed tropical and subtropical countries where sanitary conditions, hygienic practices and control of the vectors of transmission are inadequate. Because they are eukaryotes, the unicellular protozoal cells have metabolic processes closer to those of the human host than to prokaryotic bacterial pathogens. Protozoal diseases are thus less easily treated than bacterial infections. |
| |
|
Introduction to oral colon-specific drug delivery system: 2
|
| |
| The oral route is most convenient for administration of drugs to patients. Oral administration normally dissolves in the stomach fluid or intestinal fluid and absorb from these regions of the gastrointestinal tract (GIT) depends upon the physicochemical properties of the drug. Dosage forms that deliver drugs into the colon rather than upper GIT offers number of advantages. The colon is attracting interest as a site where poorly absorbed drug molecule may have an improved bioavailability. Additionally, the colon has a longer retention time and appears highly responsive to agents that enhance the absorption of poorly absorbed drugs. |
| |
| Ornidazole is readily absorbed after oral administration and bioavailability approaches 90%. Peak plasma concentrations of approximately 30 ìg/ml are achieved, usually within 2 h of single oral dose of 1.5 g which falls to about 9 mcg/ml after 24 and 2.5 mcg/ml after 48 h, Protein binding is approximately 11 to13% and Vd is 730 ml/kg. the plasma elimination half life of ornidazole is 11 to 14 h, The mean residence time is 19.4 + 0.6 h and the mean plasma clearance is 50.6 + 2.1 ml/min. Ornidazole is oxidized by the liver to a hydroxyethyl metabolite and excreted in urine.3,4 |
| |
|
1.1 Advantages of colon drug delivery system:-
|
| |
| • pH dependent system : formulation is well protected in the stomach. It has minimum side effect. |
| |
| • Unnecessary systemic absorption does not occur. |
| |
| • Colon specific formulation could be used to prolong drug delivery. |
| |
| • Enhance the absorption of poorly absorbed drug. |
| |
| • By this poorly absorbed drug molecule may have improved bioavailability. |
| |
| • It helps in efficient vaccine delivery. |
| |
MATERIALS AND METHODS
|
| |
| Ornidazole Arati Chemicals Ltd., Mumbai and Lincoln pharmaceuticals ltd., Ahmadabad. Hydroxy Propyl Methyl Cellulose K100M Colorcon Asia pvt.ltd Goa, Eudragit S 100 Unique Pharmaceuticals Laboratory Ltd. Ankleshwar, Ethyl cellulose Karnataka fine chem. Ltd. Bangalore, Dicalcium phosphate Karnataka fine chem. Ltd. Bangalore, Magnesium Stearate Karnataka fine chem. Ltd. Bangalore, Talc Karnataka fine chem. Ltd. Bangalore, Polyethylene glycol (PEG-400) Karnataka fine chem. Ltd. Bangalore, Isopropyl alcohol Karnataka fine chem. Ltd. Bangalore. |
| |
|
Drug Polymer Compatibility Studies:-
|
| |
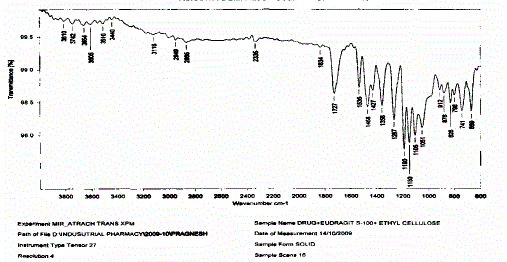
| Ø Drug polymer compatibility studies were carried out using FTIR. |
| |
| Ø The study was carried out on individual pure drug and its physical mixture with the selected polymers under study |
| |
|
UV Spectrum Analysis of Ornidazole:
|
| |
| The solution was scanned in the range of 200 to 400 nm to fix the maximum wave length and UV spectrum was obtained |
| |
|
Reagents:
|
| |
| 1) Standard Stocks – 1mg/ml in 0.1N HCl and 6.8 pH phosphate buffer. |
| |
| 2) Working Stocks – 100 ìg/ml in 0.1N HCl and 100 ìg/ml in 6.8 Ph phosphate buffer. |
| |
| 3) 0.1N HCl and 6.8 pH phosphate buffer. |
| |
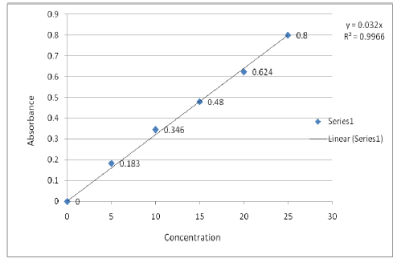
| From the working stock solutions, different aliquots of 0.5, 1, 1.5, 2.0 and 2.5 ml was taken in series of 10 ml volumetric flasks and volume was made up with 0.1N HCl and 6.8 pH phosphate buffers. The absorbance was obtained spectrophotometrically at 230 nm for 0.1N HCl and at 312 nm for 6.8 pH phosphate buffers respectively and calibration curves were constructed. |
| |
|
Method of preparation of colon targeted ornidazole tablets:
|
| |
| Ornidazole, HPMCK100/Eudragit S 100, Ethyl cellulose and Dicalcium phosphate were taken in required quantities mixed and passed through #60 sieves, lubricated with magnesium stearate and talc then was compressed into tablets in 9 mm die cavity of rotary tablet punching machine. Then film coating is done by 6% w/v solution of eudragit S 100 in isopropyl alcohol using 2% PEG-400 as plasticizer in coating pan. |
| |
EVALUATION OF COLON TARGETED TABLETS OF ORNIDAZOLE
|
| |
|
Micromeritic Properties 5
|
| |
|
Angle of Repose
|
| |
| Determined by the funnel method. The accurately 10 g weighed powder taken in a funnel. Height of the funnel was adjusted such that the tip of the funnel just touches the apex of the heap of powder. The powder was allowed to flow through funnel freely onto the surface. The diameter of the powder cone was measured and angle of repose was calculated using the following equation. |
| |
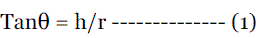 |
| |
| Therefore, θ = Tan-1h/r |
| |
| Where, θ = angle of repose, h = height of the cone r = radius of the cone base |
| |
 |
| |
|
Bulk Density
|
| |
| Both loose bulk density (LBD) and tapped bulk density (TBD) were determined. A quantity of 10 g of powder from each formulation was introduced into a 10 ml measuring cylinder. Initial volume was observed, the cylinder was allowed to tap. The tapping was continued until no further change in volume was noted. Bulk density is calculated by using formula: |
| |
| Weight of the powder |
| |
| Bulk density (ρb) = Bulk volume of the powder |
| |
| Weight of the powder |
| |
| Tapped density (ρt) = Tapped volume of the powder |
| |
|
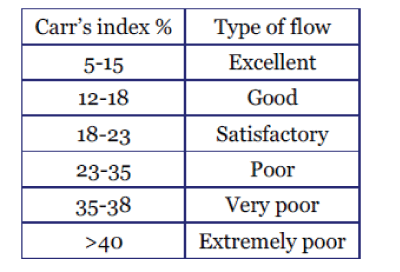
Carr’s Index
|
| |
| The carr’s index of the powder was determined by using formula: |
| |
| Carr’s index (%) = [(TBD – LBD) × 100]/TBD |
| |
| Where, LBD = weight of the powder/volume of the packing |
| |
| TBD = weight of the powder/tapped volume of the packing |
| |
 |
| |
|
Evaluation of Physicochemical Parameters of Colon Targeted Tablets
|
| |
|
Tablet Thickness 5
|
| |
| Thickness was measured using screw gauze on 3 randomly selected samples. |
| |
|
Tablet Hardness 5
|
| |
| The hardness of tablet of each formulation was measured by pfizer hardness tester. |
| |
|
Friability 5
|
| |
| Roche friabilator was used for testing the friability. Twenty tablets were weighed accurately and placed in the tumbling apparatus that revolves at 25 rpm. After 4 min., the tablets were weighed and the percentage loss in tablet weight was determined. |
| |
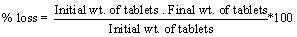 |
| |
|
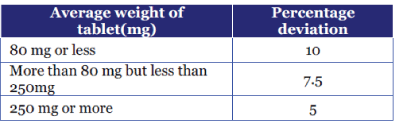
Weight Variation 5
|
| |
| Twenty tablets were weighed individually and the average weight was determined. Then percentage deviation from the average weight was calculated. Deviation should not exceed the values given in table . |
| |
 |
| |
|
Uniformity of Drug Content 6
|
| |
| Five tablets were finely powdered; quantity equivalent to 50 mg of ornidazole taken and dissolved with small amount of 6.8 pH phosphate buffer solution in to a 100 ml of volumetric flask & made up to volume with 6.8 pH buffer solution and mixed thoroughly. 1 ml withdrawn & diluted to 100 ml with 6.8 pH buffer solution and measured the absorbance at the 312 nm using a UV-visible spectrophotometer. The linearity equation obtained from calibration curve was used for estimation of Ornidazole in the tablets formulations. |
| |
|
Dissolution Studies
|
| |
| The release rate of ornidazole from tablets were determined using USP dissolution testing apparatus I (basket type). The test was performed using 900 ml of 0.1 N HCl at 37 ± 0.5°C and 100 rpm for first 2 h. then replaced with 6.8 pH phosphate buffer and continued for 24 h. Aliquot volume of 10 ml was withdrawn at regular intervals and replaced with fresh buffer diluted. The samples were replaced with fresh dissolution medium. After filtration, the amount of drug release was determined from the standard calibration curve of pure drug. |
| |
|
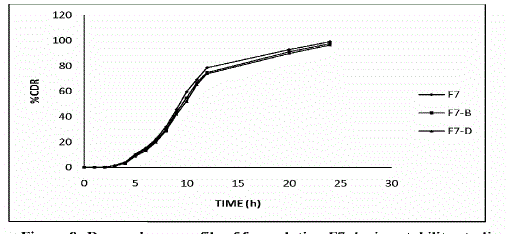
Stability Studies of the Most Satisfactory Formulation 7
|
| |
| To assess the drug and formulation stability, the stability studies were carried out of the most satisfactory formulation as per ICH guidelines. The formulation is sealed in aluminum packaging and kept in humidity chamber maintained at 30 ± 2 °C / 65 ± 5 % RH and 40 ± 2 °C / 75 ± 5 % RH for two months. At the end of studies, samples were analyzed for the drug content, in vitro dissolution, and other physicochemical parameters. |
| |
RESULTS
|
| |
|
Preformulation studies
|
| |
|
Development of standard calibration curve
|
| |
|
Standard Calibration Curve of Ornidazole In Simulated Gastric Fluid
|
| |
 |
| |
|
Standard Calibration Curve of Ornidazole In 6.8 pH Phosphate Buffer
|
| |
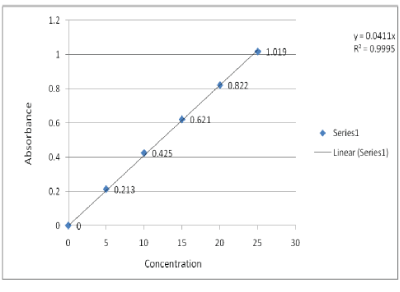 |
| |
|
Kinetics Modeling of Drug Dissolution Profiles
|
| |
| The in vitro release data obtained were fitted in to various kinetic models. Correlation coefficients of formulation F7 batch showed higher correlation with zero order plots than Higuchi and first order. So, predominant drug release mechanism is controlled release |
| |
STABILITY STUDIES
|
| |
| All values are mean of 2 readings ± standard deviation |
| |
DISCUSSION
|
| |
| The pharmacokinetic profile of ornidazole indicates that the drug is completely absorbed after oral administration. The administration of this drug in conventional tablet dosage form provides minimal amount of ornidazole for local action in the colon, still resulting in the relief of amoebiasis, but with unwanted side effects. Therefore, the targeting of Ornidazole to the colon for local action may be beneficial in avoiding the unwanted side effects as well as a lower dose of Ornidazole may be sufficient to treat amoebiasis. |
| |
|
MICROMERITIC PROPERTIES:
|
| |
|
Angle of repose
|
| |
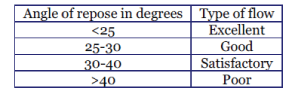
| The results of angle of repose were ranged between 25.11 ± 2.96 to 28.00 ± 1.12, which indicates good flow properties of powder. |
| |
|
Carr’s index
|
| |
| The carr’s index values were found to be in the range of 14.98 ± 0.97 % to 17.75 ± 1.29 %. These findings indicated that the powder mixture of all batches of formulation exhibited good flow properties. |
| |
EVALUATION OF PHYSICOCHEMICAL PARAMETERS
|
| |
|
Tablet Thickness
|
| |
| Thickness of the developed formulations F1 to F8 varied from 5.22 ± 0.021 mm to 5.45 ± 0.017 mm. |
| |
|
Tablet Hardness
|
| |
| Hardness of the developed formulations F1 to F8 varied from 7.5 ± 0.34 kg/cm2 to 9.9 ± 0.26 kg/cm2. |
| |
|
Friability
|
| |
| Friability of the developed formulations varied from 0.21 ± 0.03 % to 0.47 ± 0.035 % loss which was less than 1% as per official requirement of IP. |
| |
|
Weight Variation before coating
|
| |
| The average weight of twenty tablets was calculated for each formulation which varied from 399.91 ± 0.53 mg to 400.98 ± 0.57 mg that complied the official requirement as per IP. |
| |
|
Weight Variation after coating
|
| |
| The average weight of twenty tablets was calculated for each formulation which varied from 406 ± 0.34 mg to 408 ± 0.23 mg. |
| |
|
Uniformity of Drug Content
|
| |
| The drug content varied from 98.26 ± 1.03 % to 100.00 ± 0.69 % which was within the required limits. |
| |
IN VITRO DRUG RELEASE STUDIES
|
| |
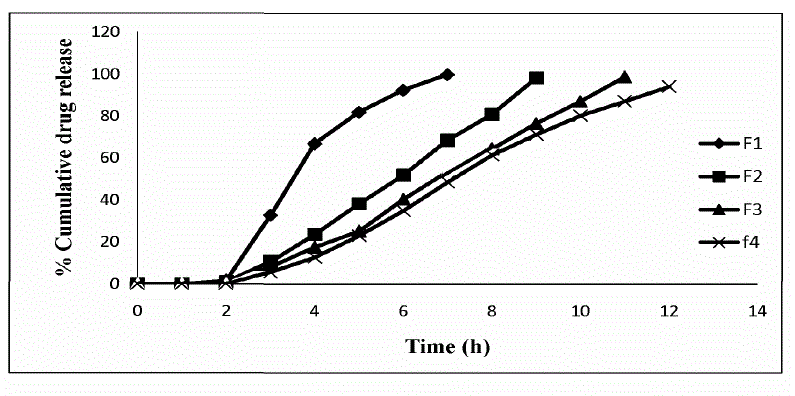
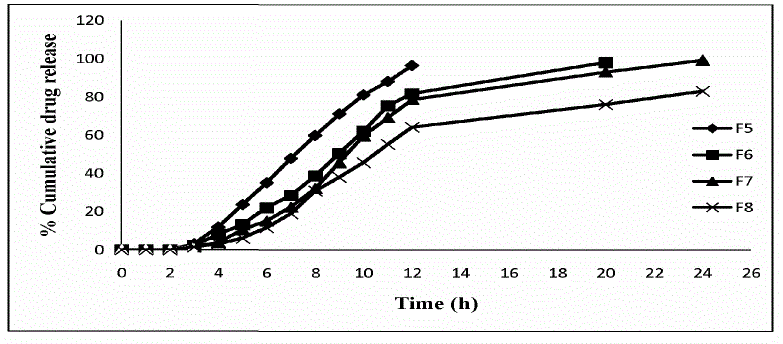
| The release of ornidazole from colon targeted tablets varied according to the types and proportion of matrix forming polymers. |
| |
|
HPMC K100M and Ethyl cellulose based formulations
|
| |
| The progressive decrease in the amount of drug release from formulations F1 to F4 attributed to gradual increase in HPMC K100M and Ethyl cellulose contents. The duration of drug release was slower with formulation F4 which was about only 94.18 ± 1.68 % in 12 h from among the formulations F1 to F4. |
| |
|
Eudragit S100 and Ethyl cellulose based Formulations
|
| |
| The progressive decrease in the amount of drug release from formulations F5 to F8 attributed to gradual increase in Eudragit S100 and Ethyl cellulose contents. The duration of drug release was slower with formulation F8 which was about only 83.20 ± 0.18 % in 24 h from among the formulations F5 to F8. |
| |
KINETICS MODELING OF DRUG DISSOLUTION PROFILES
|
| |
| The in vitro release data obtained were fitted in to various kinetic models. Correlation coefficients of formulation F7 batch showed higher correlation with zero order plots than Higuchi and first order. So, predominant drug release mechanism is controlled release. |
| |
STABILITY STUDIES
|
| |
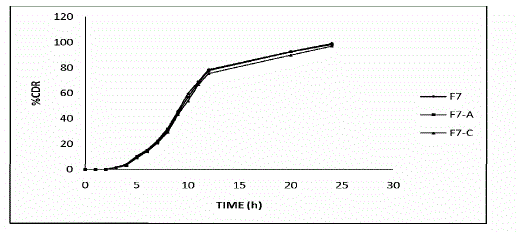
| Stability studies were carried out of the most satisfactory formulation F7 at 30 ± 2 °C / 65 ± 5 % RH and 40 ± 2 °C / 75 ± 5 % RH for two months as per ICH guidelines. At various time intervals of 30 days and 60 days end, samples were evaluated. There was no major change in the various physicochemical parameters evaluated. |
| |
CONCLUSION
|
| |
| ØThe present investigation was concerned with the development of the colon targeted tablets, which after oral administration were designed to prevent the drug release in stomach and small intestine. It improves the bioavailability of the drug as well as its half life. |
| |
| Ø Various formulations were developed by using release rate controlling polymers like HPMC K100M, Eudragit S100, Ethyl cellulose by direct compression method. |
| |
| Ø Developed film coated colon targeted tablets possessed the required physicochemical parameters such as hardness, friability, weight variation, drug content. |
| |
| Ø The most satisfactory formulation (F7) had showed no significant change in physicochemical properties, drug content, in vitro dissolution pattern after storage at 30 ± 2 °C / 65 ± 5 % RH and at 40 ± 2 °C / 75 ± 5 % RH during stability studies for two months. Ø Therefore, it was concluded that the most satisfactory formulation is (F7) |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
 |
 |
 |
 |
| Table 6 |
Table 7 |
Table 8 |
Table 9 |
|
| |
Figures at a glance
|
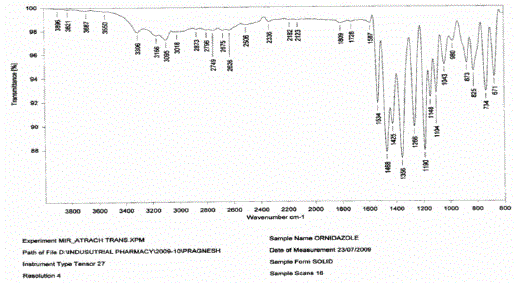 |
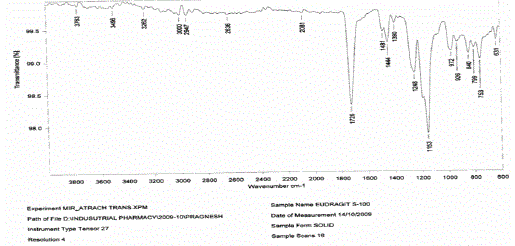 |
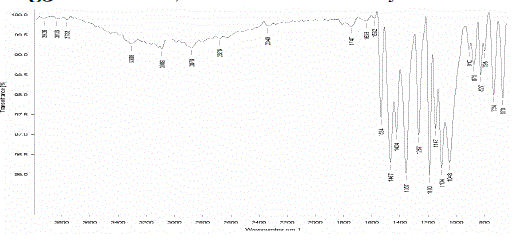 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |
 |
 |
 |
 |
| Figure 5 |
Figure 6 |
Figure 7 |
Figure 8 |
|
| |