&nbs
Key words
|
| |
| Ketorolac, in situ gel, Differential scanning calorimetry, in vitro diffusion study, Draize test. |
| |
INTRODUCTION
|
| |
| The available drug delivery systems are fairly primitive and inefficient. Medication is applied to the surface of eye for two purposes, to treat the outside of eye for infection (conjunctivitis) or to provide intraocular treatment through the cornea for diseases (glaucoma). Most ocular diseases are treated with a topical application of solution into the lower cul-de-sac as eye drop [1]. Eye drops that are conventional ophthalmic delivery systems often result in poor bioavailability and therapeutic response due to high tear fluid turnover and dynamics that cause rapid precorneal elimination of the drug. A high frequency of eye drop instillation is also associated with patient non-compliance. Inclusion of excess drug in the formulation to overcome bioavailability problems is associated with side effects issues if the drug solution drained from the eye is systemically absorbed from the nasolacrimal duct. One way of prolonging the availability of drug in the precorneal area involves increasing the viscosity of the dosage form by adding water-soluble polymers. Here alternative approach was aimed to increase precorneal residence time of drugs that the polymeric solutions are change in in-situ gel because of pH, physiological temperature and ionic composition of the lachrymal fluid. Ketorolac tromethamine (KETOROLAC) is a potent and effective aryl-acetic acid NSAID and is nonirritating to the eye at 0.5% w/v concentration [2]. Aqueous ocular drops of ketorolac are effective and safe for topical use following cataract surgery and intraocular lens implantation [3]. Ketorolac is also a viable alternative to corticosteroids in treating ocular inflammation in the presence of pathogens[4]. Ophthalmic solutions of ketorolac (0.5%) are effective in the treatment of chronic aphakic and pseudoaphakic macular edema [5]. The topical ophthalmic dose of ketorolac is 1 drop qid in allergic conjunctivitis and in cystoids macular edema. The objective of the present work was to develop a pH-triggered in situ gelling system for sustained ophthalmic delivery of ketorolac and simultaneously determine the rheology behaviour, characterisation of gel. A combination of Carbopol (940) and hydroxyl propyl- methyl cellulose (HPMC K15M, K4M) was used as vehicle for the formulation of eye drops of ketorolac (0.5%, w/v) that would gel when instilled into the eye, and provide sustained release of ketorolac during treatment of seasonal allergic conjunctivitis and in some ocular inflammation situations[6]. |
| |
|
Materials:
|
| |
| Ketorolac was a gift sample from Ranbaxy Laboratories Limited, (Gurgaon, India). Carbopols 940 and HPMC K15M, K4M (Methocel) purchased from SD fine-chem. Ltd, Mumbai. All other reagents used were of analytical grade. |
| |
|
Methods:
|
| |
|
Differential scanning calorimetry (DSC) characterization
|
| |
| Thermal characterization of pure drug and physical mixture was performed with Calor imeter . Samples were weighed (2.00 ± 0.5mg) and placed in sealed aluminium pans. The equipment was calibrated with indium. The samples were scanned at 20°c / min from 25°c to 300°c. |
| |
|
Selection of vehicle:
|
| |
| The solubility of ketorolac was evaluated in various buffers, e.g., acetate buffer I.P. (pH 4.6, 4.8, 5.0, 5.5, and 6.0), citrophosphate buffer B.P. (pH 5.0. 6.0. 6.2 and 7.0) and phosphate buffer USP (pH 5.5, 6.0, 6.5, and 7.2) to select a suitable vehicle [7]. Solutions of ketorolac (0.5%, w/v) in the buffers in which it was soluble were prepared and tested for stability to light, temperature, and autoclaving. Solubility of ketorolac was evaluated using a U.V. spectrophotometer at 321 nm[8]. |
| |
|
Preparation of In-situ gelling system:
|
| |
| Aqueous solutions of varying concentrations of Carbopols 940 (CP) and HPMC of different grades (formulation codes K 1, K 2, K 3 ... K 28) were prepared and evaluated for gelling capacity and viscosity in order to identify the compositions suitable for use as in situ gelling systems (Table 1). The gelling capacity was determined by placing 1 drop of the formulation in a vial containing 2ml of artificial tear fluid freshly prepared and equilibrated at 37°c and visually assessing gel formation and noting the time for gelation and the time taken for the gel formed to dissolve. The composition of artificial tear fluid used was NaCl 0.670 g, sodium bicarbonate 0.200 g, calcium chloride -2 H2O 0.008 g, purified water q.s. 100.0 g[9]. The viscosity at was measured using a Brookfield viscometer (DVULTRA model) in a small volume adapter used for purposes of comparative evaluation. |
| |
| The detailed procedure for preparing the in situ gelforming system of ketorolac is outlined below Table 2. Buffer salts were dissolved in 75 ml of purified water, HPMC K15M, K4M was added and allowed to hydrate. Carbopols940 was sprinkled over this solution and allowed to hydrate overnight. The solution was stirred with an overhead stirrer, Tween 80 was added while stirring. Ketorolac was dissolved in purified water, benzalkonium chloride (BKC) was then added and the solution was filtered through 0.2-µm cellulose acetate membrane filter. The drug solution was added to the Carbopol - HPMC solution under constant stirring until a uniform solution was obtained. Purified water was then added to make up the volume to 100 ml. The developed formulations were filled in 5-ml capacity amber glass vials, closed with gray butyl rubber closures and sealed with aluminium caps. The formulations, in their final pack were subjected to terminal sterilization by autoclaving at 121°c and 15 psi for 20 min[7,10]. |
| |
|
Evaluation of formulation:
|
| |
| The general appearance of the formulations was observed which included colour and clarity of solution. The pH of the prepared formulations was checked using a pH meter. The Gelling capacity of the formulations was evaluated for gelling property in order to identify the formulations suitable for use as in situ gelling systems. Gelling was determined by mixing the formulation with simulated tear fluid in the proportion 25:7 and the gelation was assessed by visual examination. The time taken for gel to form and the time taken for it to dissolve was noted. The drug content was determined [10] by taking 1ml of the formulation and diluting it to 100ml with distilled water. Aliquot of 5ml was withdrawn and further diluted to 25ml with distilled water. Ketorolac concentration was determined at 321 nm by using UV-Visible spectrophotometer. Viscosity of the instilled formulation is an important factor in determining residence time of drug in the eye. |
| |
|
Rheological Studies:
|
| |
| The developed formulation (pH 6.0) was poured into the small sample adaptor of the Brookfield viscometer DU ULTRA+ rheometer with UL adopter, spindle 00, and the angular velocity was increased gradually from 2 to 50 rpm. The hierarchy of the angular velocity was reversed. The average of the two readings was used to calculate the viscosity. The formulation was then poured into an ointment jar and the pH raised to 7.4 by adding 0.5 M NaOH by hallipath spindle. The rheology of the resultant gel was studied using T bar F. |
| |
|
In-Vitro Drug Release Studies:
|
| |
| The in-vitro release of Ketorolac from the prepared formulations was studied through cellophane membrane using a modified USP XXIII dissolution testing apparatus (open cylinder method). The dissolution medium used was artificial tear fluid freshly prepared of pH 7.4.buffer Cellophane membrane previously soaked overnight in the dissolution medium was tied to one end of a specifically designed glass cylinder (open at both ends of 5 cm diameter) a 2 ml volume of the formulation was accurately pipette in to this assembly. The cylinder was attached to the metallic drive shaft and suspended in 100 ml of dissolution medium maintained at 37±1°c so that the membrane just touched the receptor medium surface. The shaft was rotated at 50-rpm aliquots each of 1 ml volume, was withdrawn at hourly intervals and replaced by an equal volumes of the receptor medium. The aliquots were diluted with receptor medium and absorbance was measured at 321 nm. The in vitro release studies were also carried out for the marketed conventional ophthalmic drops of ketorolac (0.5% w/v) in order to compare the release profile of the drug with the prepared in situ gelling system [11]. Sterilization of the selected formulations was carried out by the sterility testing was performed (IP, 1996). |
| |
|
Ocular irritancy:
|
| |
| The Draize technique was designed for the ocular irritation potential of the ophthalmic product prior to marketing. According to the Draize test, the amount of substance applied to the eye is normally 100ìl placed into the lower cul-de-sac with observation of the various criteria made at a designed required time interval of 1hr, 24hrs, 48 hrs, 72hrs, and 1week after administration. Three rabbits (male) weighing 1.5 to 2kg were used for the present study. The sterile formulation was instilled twice a day for a period of 7 days, and a cross-over study was carried out (a 3 day washing period with saline was carried out before the cross-over study). Rabbits were observed periodically for redness, swelling, watering of the eye. The results are as shown in table 4. [12]. |
| |
RESULTS AND DISCUSSION:
|
| |
|
Differential Scanning Calorimetry (DSC):
|
| |
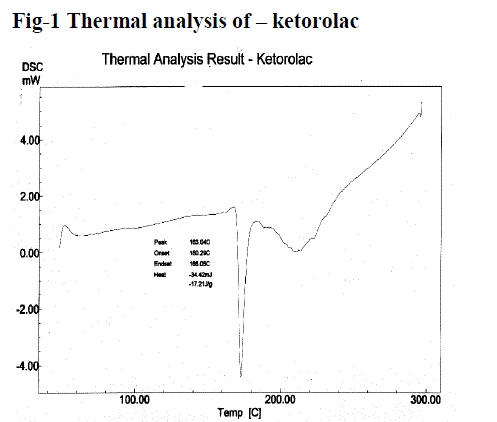
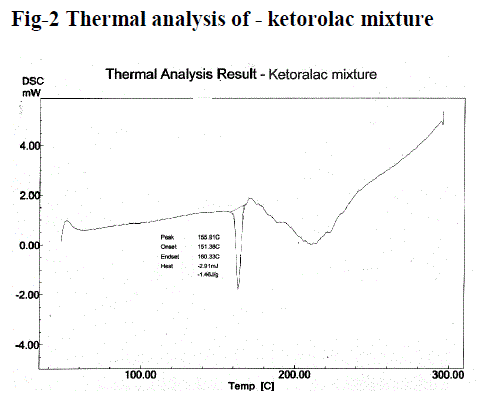
| Figure no. 1 and 2, compares the DSC thermo gram of Ketorolac and physical mixture of Ketorolac. Ketorolac showed a long and sharp characteristic endothermic peak at 163.04°c due to its phase transition system. The physical mixture of Ketorolac with Carbopol and HPMC shows characteristic peak at 155.91°c. It showed the slight change in characteristic peak may be due to fusion of excipient present in the Physical mixture. From this result, it clears that there is no interaction in between Ketorolac and excipients. |
| |
|
Selection of vehicle:
|
| |
| Buffers play a pivotal role in formulating ophthalmic drops contributing significantly to chemical stability and clinical response and also influencing the comfort and safety of the product, hence the importance of selecting a suitable buffer ensures product stability and desired drug solubility. The studies in various buffer solutions indicated the drug was soluble in acetate buffers of pH 5.0, 5.5, and 6.0, citrophosphate buffers of pH 5.0, 6.0, 6.2, and 7.0 and phosphate buffers of pH 5.5, 6.0, 6.5 and 7.2 at the dosage level desired (0.5%, w/v). The solutions were stable to elevated temperatures and autoclaving. However, their instability to light as evidenced by discoloration of the exposed solutions necessitated their packing in amber vials. Citrophosphate buffer, pH 6.0, was selected as a vehicle for the formulated in situ gelling systems as the ketorolac precipitates at a pH value of ?5.0, at the dosage level desired; it is easily neutralized by the buffering action of the tear fluid. |
| |
|
Preparation of formulations
|
| |
| The use of carbopol (polyacrylic acid, PAA) in insitu gel-forming systems is substantiated by the property of its aqueous solutions to transform into stiff gels when the pH is raised. However, the concentration of PAA required forming stiff gels results in highly acidic solutions that are not easily neutralized by the buffering action of the tear fluid. A reduction in PAA concentration without compromising the gelling capacity and rheological properties of the delivery system may be achieved by the addition of viscosity-enhancing polymers such as HPMC. The two main prerequisites of an in situ gelling system are viscosity and gelling capacity (speed and extent of gelation). The formulation should have an optimum viscosity that will allow easy instillation into the eye as a liquid (drops), which would undergo a rapid sol-to-gel transition (triggered by a rise in pH from 6.0 to 7.4). Additionally, to facilitate sustained release of drug to the ocular tissue, the gel formed in situ should preserve its integrity without dissolving or eroding for a prolonged period of time. Table 1 shows the gelling capacity and of formulations K 1–K 28. |
| |
| A concentration of 0.4% and 0.6% HPMC K15M and 0.4% , Carbopol 940(code K 10, K11) and concentration HPMC K4M 0.5% and 0.3%, and 0.4% and 0.5% carbopol 940 (code K24, K26) respectively was selected as it had satisfactory attributes of viscosity and gelling capacity. Ketorolac was dissolved in purified water, benzalkonium chloride (BKC 0.01%) was incorporated as a preservative and the solution was filtered through 0.2-µm cellulose acetate membrane filter. The formulae for K 10, K11, K 24, K 26, are listed in Table 2. Carbopol being acidic in nature, with a pH of 1% solution, is 2.5–3, lowered the pH of the formulation prepared with citrophosphate buffer B.P. of pH 6.0–5.2. As the Ketorolac precipitates at the pH below 5.0 at the dosage level desired, the pH of the final formulation was adjusted to 6.0 with 0.5M sodium hydroxide solution. Since the ingredients themselves contributed to the tonicity, no tonicity adjusting agents were added. BKC (0.01%, w/v) was incorporated as a preservative in both formulation code K 10, K11, K24 and K28. |
| |
|
Evaluation of formulations:
|
| |
| The clarity, pH and drug content of the formulations were found to be satisfactory (Table 3). The formulations were liquid at room temperature and at the pH formulated (pH 6.0) and underwent rapid transition in to the gel phase at the pH of the tear fluid (pH 7.4). Terminal sterilization by autoclaving had no effect on clarity, pH, viscosity, and gelling capacity of K 10, K11, K24 and K 26. The haziness observed after autoclaving (due to precipitation of HPMC at elevated temperature) was found to disappear, and the original clarity was regained after overnight standing. |
| |
|
Rheological Study:
|
| |
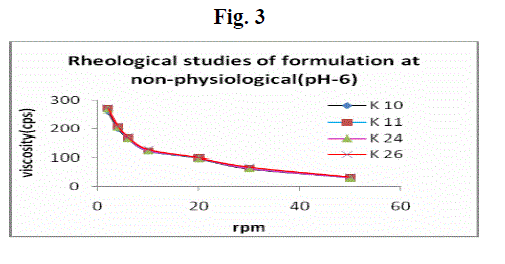
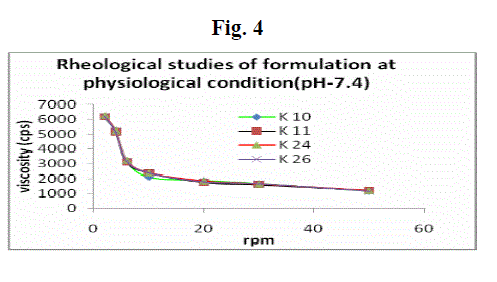
| Formulations were shear thinning and an increase in shear stress was observed with increase in angular velocity (pseudo-plastic rheology) (Fig. 3 and 4). The administration of ophthalmic preparations should influence as little as possible the pseudoplastic character of the precorneal tear film. Since the ocular shear rate is very high, ranging from 0.03 s-1 during interblinking periods to 4250–28,500 s-1 during blinking viscoelastic fluids with a viscosity that is high under low shear rate conditions and low under the high shear rate conditions are often preferred. At pH 6.0, the formulations were in a liquid state and exhibited low viscosity. An increase in pH to 7.4 (the pH of the tear fluid) caused the solutions to transform into gels with high viscosity. |
| |
|
In-vitro drug release:
|
| |
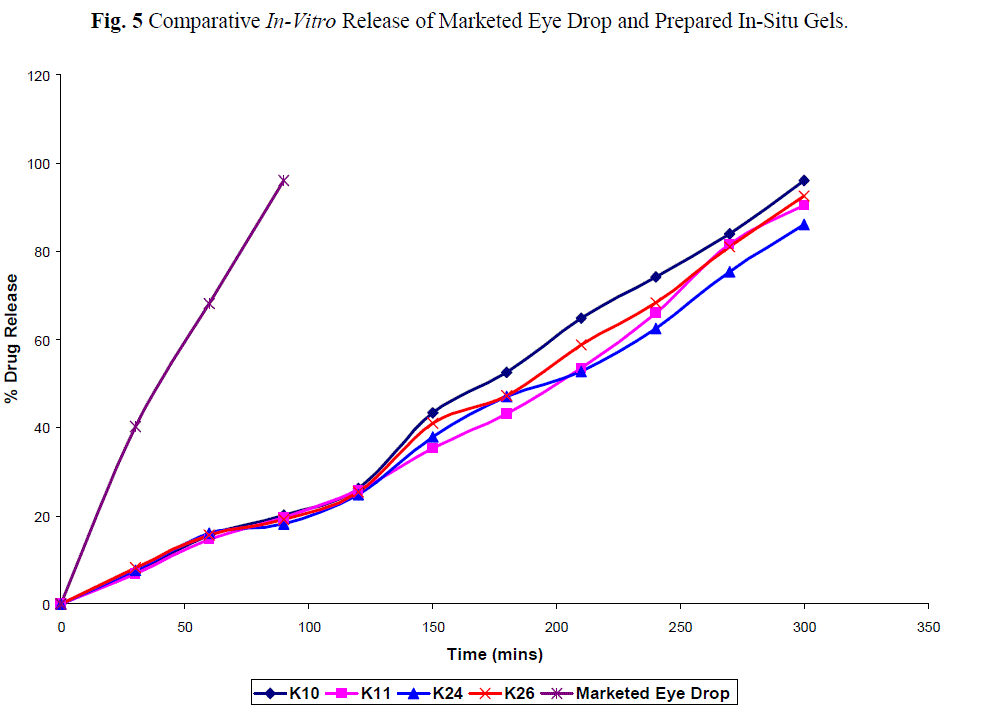
| The cumulative percent of ketorolac released as function of time is show in fig 5. Addition of Tween 80 did not alter the release profile of ketorolac. All four formulation (K 10, 11, 24, 26) sustained drug release for 8 hours, which may be due to the higher concentration of carbopol with HPMC. |
| |
| The comparative in vitro drug release profile fig. 3 between the marketed conventional ophthalmic drops and the all formulation showed 95% and 7.1%, 6.9%, 7.6% and 8.2% after initial 30min. At the end of 90 min. the drug release was found to be 95.92% and 20.1%, 19.6%, 18.1% and 19.2% from the marketed product and K 10, K11, K24, K26 indicating that the drug release was significantly prolonged by using the in situ gelling systems. The all four formulation passed the sterility test as there was no appearance of turbidity and hence no evidence of microbial growth when incubated for not less than 14 days at 30-35oC in case of fluid thioglycollate medium and at 20-25oC in the case of soyabean casein digest medium. |
| |
|
Ocular irritancy:
|
| |
| In-vivo eye irritation testing was carried out using rabbits and as per Draize test protocol. Optimized two formulations K11 & K26 were used for this test. The formulations were found to be non-irritating with no ocular damage or abnormal clinical signs to the cornea, iris or conjunctivae observed. Hence the formulation was suitable for the eye instillation. |
| |
CONCLUSION:
|
| |
| Ketorolac is a potent and effective aryl-acetic acid NSAID and is nonirritating to the eye, was successfully formulated as pH triggered in-situ gel forming eye drops(0.5%w/v) using carbopol 940 as a gelling agent in combination with HPMC (K15M/K4M) as a viscosity enhancing agent. The formulation was liquid at the formulated pH (6.0) and underwent rapid gelation upon raising the pH to 7.4. The gel formed in situ afforded sustained drug release over an 8-hour period. The developed formulation is a viable alternative to conventional eye drop by virtue of its ability to enhance bioavailability through its longer precorneal residence time and ability of sustain drug release. Clarity of all the formulations was found to be satisfactory. Terminal sterilization with autoclaving had no effect on the physicochemical properties of the formulations. The pH was within acceptable range and formulation does not cause any reduction upon administration of the formulation in rabbit eye during the draize test. The in-vitro release studies were carried out for all formulations using STF as the dissolution medium. The data of these studies and results indicates that (K-10, 11, 24, 26) showed better sustaining effect amongst all formulations. |
| |
|
Conflict of Interest
|
| |
| None |
| |
|
Source of Support
|
| |
| Nil |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
|
References
|
- Martin RG, Jolly RP, Megha B, Dharmesh MM. A pH-triggered In situ gel-forming ophthalmic drug delivery system for tropicamide. Drug Delivery technology 2007; 44-49.
- Malhotra M, Mujumdar DK. In vivo ocular availability of ketorolac following ocular instillations of aqueous, oil, and ointment formulations to normal corneas of rabbits: A technical Note. AAPS Pharmasci 2006; E1-E6.
- Flach AJ, Kraff MC, Sanders DR, Tanenbaum L. The quantitative effect of 0.5% ketorolac tromethamine solution and 0.1% dexamethasone sodium phosphate solution on post surgical blood aqueous barrier. Arch Ophthalmol 1988; 106:480-483.
- Fraser-Smith EB, Mathews TR. Effect of ketorolac on pseudomonas aeruginosa ocular infection in rabbits. J OculPharmacol 1988; 4:101-109.
- Flach AJ, Dolan BJ, Irvine AR. Effectiveness of ketorolac tromethamine 0.5% ophthalmic solution for chronic aphakic and pseudoaphakic cystoid macular edema. Am J Ophthalmol 1987; 103: 479-486.
- Manjappa AS, Basavaraj K, Nanjwade, Manvi FV, Murthy RSR. Sustained ophthalmic in situ gel of ketorolac tromethamine rheology and in vivo Studies. Drug Development Research 2009; 70: 417-424.
- Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release 2001; 73:205-211.
- Arora R, srivastava AK, Rani M, Mishra B. Investigation on osmotic matrix tablets as controlled delivery system of ketorolac tromethamine. Acta pharmaceutical turcica 2002; 44; 87-96.
- Chunjie WU, Hongyi QI, Chen W, Huang C. Preparation and evaluation of a carbopol /HPMC-based in situ gelling ophthalmic system for puerarin. The pharmaceutical society of Japan 2007; 127(1)183-191.
- Abraham S, Furtado S, Bharath S, Basavaraj BV, Deveswaran R, Madhavan V. Sustained ophthalmic delivery of ofloxacin from an ionactivated in situ gelling system. Pak. J. Pharm. Sci 2009; 175 - 179.
- Mohan EC, Kandukuri JM, Allenki V. Preparation and evaluation of in-situ gels for ocular drug delivery. Journal of Pharmacy Research 2009, 2(6), 1089-1094.
- Hiremath SSP, Dasankoppa FS, Nadaf A, Jamakandi VG, Mulla JS, Sreenivas SA, Sholapur HN, Aezazahmed, Nanjundaswamy NG. Formulation and evaluation of a novel in situ gum based ophthalmic drug delivery system of linezolid. Sci pharm 2008; 76: 515-532.
|
p; |
















