Key words
|
| |
| Aceclofenac; topical gel; carbopol; hydroxypropylmethylcellulose; carboxymethylcellulose; sodium alginate; penetration enhancer; drug release |
| |
Introduction
|
| |
| Delivery of drugs to the skin is an effective and targeted therapy for local dermatological disorders. This route of drug delivery has gained popularity because it avoids first pass effects, gastrointestinal irritation, and metabolic degradation associated with oral administration [1]. Due to the first past effect only 25-/45% of the orally administered dose reaches the blood circulation. In order to bypass these disadvantages the gel formulations have been proposed as topical application [2]. Topical gel formulations provide a suitable delivery system for drugs because they are less greasy and can be easily removed from the skin. Percutaneous absorption of drugs from topical formulations involves the release of the drug from the formulation and permeation through skin to reach the target tissue. The release of the drug from topical preparations depends on the physicochemical properties of the vehicle and the drug employed. In order to enhance drug release and skin permeation, methods such as the selection of a suitable vehicle5, co-administration of a chemical enhancer [3] have been studied. Gel base formulation makes the drug molecules more easily removable from the system then cream and ointment [4, 5]. Gels for dermatological use have several favorable properties such as being thixotropic, greaseless, easily spreadable, easily removable, emollient, nonstaing, compatible with several excipients and water-soluble or miscible [6]. |
| |
| Aceclofenac is chemically [[[2- [(2,6,Dichlorophenyl) amino] phenyl] acetyl] oxy] acetic acid [7], is a new orally effective NSAID of the phenyl acetic acid group. It posses a remarkable antiinflammatory, analgesic and antipyretic properties. The continue use of aceclofenac through oral route causes ulcerogenic effect, flatulence, indigestion (dyspepsia), vertigo, dizziness, dyspnoea, stomatitis, itching (pruritis) [8, 9]. When a drug system is applied topically, drug diffuses passively out of its carrier or vehicle. A unique feature of aceclofenac’s pharmacology is that it stimulates glycosaminoglycans (GAG) synthesis, which in turns enhances skin permeation of NSAIDs [10]. Aceclofenac when presented in the form of topical gel can reduce local inflammations. Hence for local inflammation or pain in the body, the topical application of aceclofenac may be useful which also avoids the side effects associated with the oral therapy. Hence, a topical ointment containing aceclofenac was prepared [11]. It is established that gel formulations are superior topical formulation over any other topical formulations, because these system have better application property in comparison to creams and ointments [12]. |
| |
| The objective of present study was conducted to develop a gel formulation of aceclofenac using four types of gelling agents: carbopol, hydroxypropylmethylcellulose (HPMC), carboxymethylcellulose sodium (Na CMC) and sodium alginate. Effect of penetration enhancer (propylene glycol) on the release has been studied. The gels were evaluated for physical appearance, rheological behavior, drug release and stability. The drug release from all gelling agents through a standard cellophane membrane was evaluated using Keshary-Chien diffusion cell. |
| |
MATERIALS
|
| |
| Aceclofenac (Gift sample, Aristo Pharma Pvt Ltd, Bombay), carbopol-934, Na CMC salt medium viscosity 200-400 cPs, sodium alginate, propylene glycol, triethanolamine, propylparaben, sodium hydroxide, potassium di hydrogen ortho phosphate, HPMC, Methylparaben (N R Chem, Bombay). |
| |
EQUIPMENT
|
| |
| Digital balance (Shimadzu Corporation, Japan), UV-Visible spectrophotometer (UV-1700A Shimadzu corporation, Japan), pH meter, Magnetic stirrer, Water bath shaker (Servewell Instruments and Equipments Pvt. Ltd.Banglore, India), Brookfield LVDV-II +Pro Viscometer (Brookfield Engineering Laboratories, Inc. USA). |
| |
METHODS
|
| |
|
Preparation of gels:
|
| |
| Appropriate quantity of carbopol 934was soaked in water for a period of 2 hours. Carbopol was then neutralized with triethanolamine (TEA) with stirring. Then specified amount of drug was dissolved in appropriate and preweighted amounts of propylene glycol and ethanol. Solvent blend was transferred to carbopol container and agitated for additional 20 min. The dispersion was then allowed to hydrate and swell for 60 min, finally adjusted the pH with 98% TEA until the desired pH value was approximately reached (6.8-7). During pH adjustment, the mixture was stirred gently with a spatula until homogeneous gel was formed. All the samples were allowed to equilibrate for at least 24 hours at room temperature prior to performing rheological measurements [13-18]. |
| |
| Other gel formulations were prepared by dispersing 3% w/w HPMC, 5% w/w Na CMC and 8% w/w sodium alginate in water by continuous stirring for a period of 2 h. Aceclofenac was dissolved in propylene glycol or ethanol or isopropyl alcohol and the solution was added gently to HPMC, Na CMC and sodium alginate dispersion under continuous stirring. The mixture was stirred gently with a spatula until homogeneous gel was formed. All the samples were allowed to equilibrate for at least 24 h at room temperature prior to performing rheological measurements [19-23]. |
| |
|
Physical examination [24]
|
| |
| The prepared aceclofenac gels were inspected visually for their color, homogeneity, consistency, spreadability and phase separation. The pH was measured in each gel, using a pH meter, which was calibrated before each use with standard buffer solutions at pH 4, 7, 9. The electrode was inserted in to the sample 10 min priors to taking the reading at room temperature |
| |
|
Drug content studies [25]
|
| |
| To ensure uniform formulation of the gel, it was sampled from the different locations in the mixer and assayed for the drug content. Drug content of the gels was determined by dissolving an accurately weighed quantity of gel (about 1 gm) in about 100 ml of pH 6.8-phosphate buffer. These solutions were quantitatively transferred to volumetric flasks and appropriate dilutions were made with the same buffer solution. The resulting solutions were then filtered 0.45 mm membrane filters before subjecting the solution to spectrophotometric analysis for aceclofenac at 274 nm. Drug content was determined from the standard curve of aceclofenac. |
| |
|
In vitro release study of aceclofenac
|
| |
| Release of aceclofenac from various gel formulations was studies using a modified Keshary- Chien diffusion cell. A standard cellophane membrane (soaked in pH 6.8 for two hours before use) was fixed to one end of the cylinder with the aid of an adhesive to result in permeation cell. One gram of gel was taken in the cell (donor compartment) and the cell was immersed in beaker (100 ml) containing drug free phosphate buffer pH 6.8 (90 ml) as receptor compartment. The cell was immersed to a depth of 1 cm below the surface of phosphate buffer in the receptor compartment and was agitated using a magnetic stirrer and a temperature of 32 °C ± 1°C was maintained. Sample (5 ml) of the receptor compartment was taken at various interval of time (30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330, 360 min) over a period of 6 hours and assayed for aceclofenac at 274 nm. The volume withdrawn at each time was replaced with drug free phosphate buffer. Amount of aceclofenac released at various intervals of time was calculated and plotted against time. |
| |
| A viscometer (Brookfield digital viscometer DV II RVTDV-II USA) was used to measure the viscosities (in cPs) of the gels. The spindle (TF 96) was rotated at 0.5 rpm. Samples of the gels were to settle over 30 min at the assay temperature (25 ±/1 °C) before the measurements were taken. |
| |
|
Stability study
|
| |
| For the evaluation of stability study, maintaining the formulations at an ambient condition over a period of two months. The physical appearance, pH value, drug content, rheological properties, drug release studies were determined periodically after the 1st and 2nd month after topical gel preparations. |
| |
RESULTS AND DISCUSSION
|
| |
|
Physical examination
|
| |
| The prepared aceclofenac gel formulations were transparent in carbopol 934, white viscous in HPMC or Na CMC and brownish gummy in sodium alginate with a smooth and homogeneous appearance. They were easily spreadable with acceptable (Table 4,7,10). The pH values of all the prepared formulations ranged from 5 to 6.8, which is considered acceptable to avoid the risk of irritation upon application to the skin.[27, 28]. |
| |
|
Effect of polymer concentration on drug release
|
| |
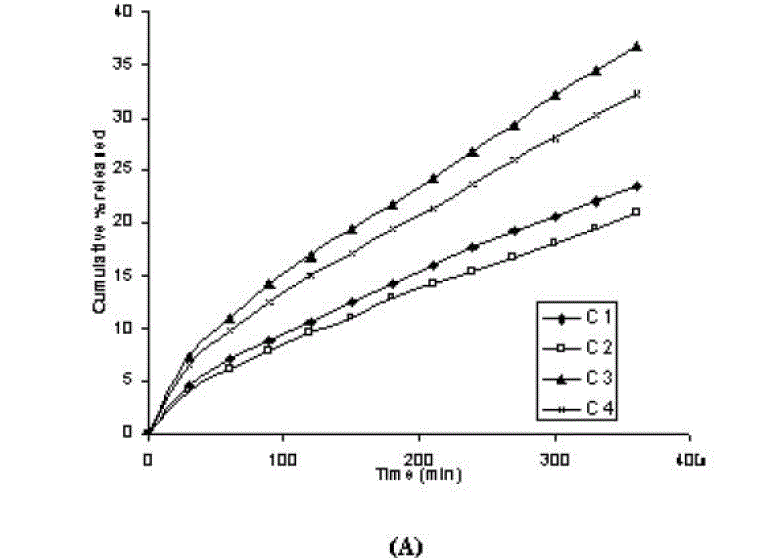
| In vitro dissolution profile of aceclofenac gels containing different concentration of carbopol (1% and 2%) are shown in figure 1 (C 1 and C 2). the initial concentration of aceclofenac in all of the gels was kept as constant at 1.5%. It is clear from the Table 2 that drug release at 180 minute or 360 minute decreased (p<0.05) with increase in the concentration of the carbopol. The steady-state flux (permeability coefficient and drug release) values were also found lower for the formulation in which polymer concentration was kept high (Table 3). In addition, viscosity increased (from 152 x 103 cPs to 447 x 103 cPs, Table3) as polymer concentration increased. Viscosity is negatively related to the release of active substance from formulations and its penetration through the diffusion barriers. The decrease in the release could be attributed to increased microviscosity of the gel by increasing polymer concentration. Thus, both high concentration of polymer and high viscosity compete each other in decreasing the release of active substance from the formulation [29, 30]. In our study, the finding that higher polymer concentration resulted in lower drug release from the vehicles is in agreement with Lauffer’s molecular diffusion theory of polymer gels [31], which states that the diffusion coefficient of a solute is inversely proportional to the volume fraction occupied by the gel-forming agent. |
| |
|
Effect of penetration enhancer
|
| |
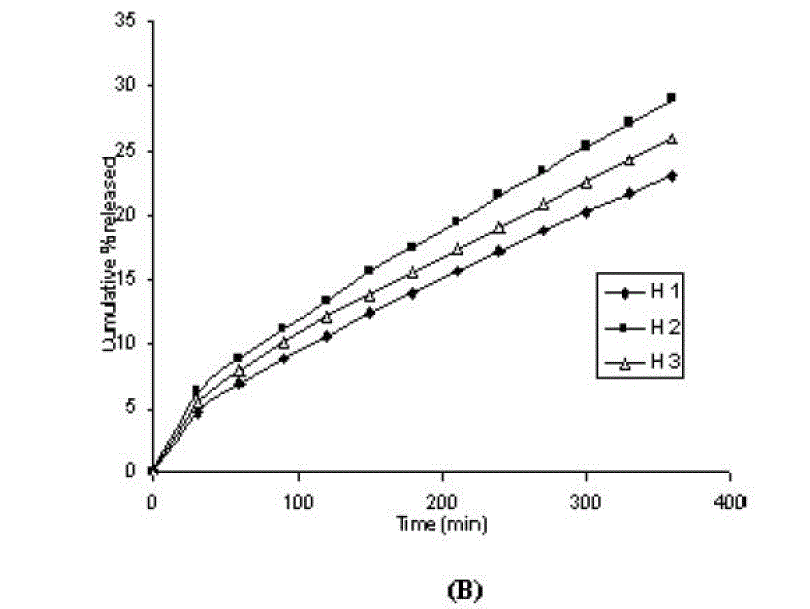
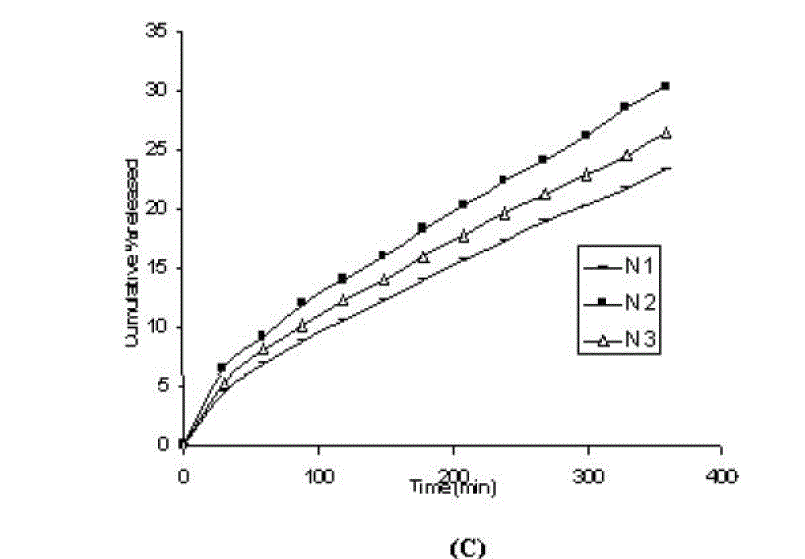
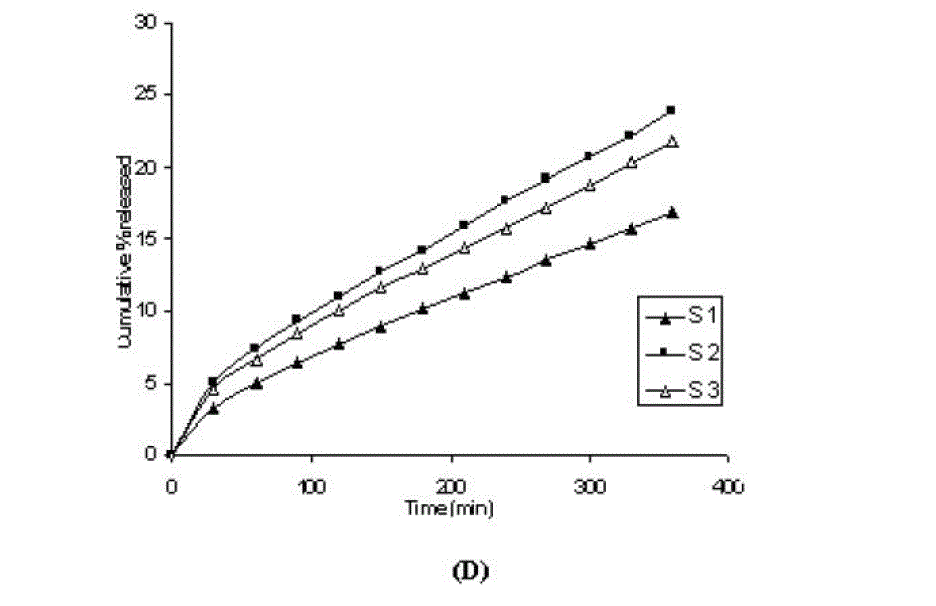
| One of the approaches for improving the topical drug delivery uses the penetration enhancer. In this connection, numerous compounds have been evaluated. Among them, propylene glycol has been the most commonly used excipient in topically applied dosage forms. In this study, effect of the concentration of propylene glycol (5 and 10% v/v) on the permeation of aceclofenac has been studied. The concentration of propylene glycol increased keeping the concentration of polymer constant (carbopol 1%, HPMC 3%, Na-CMC 5% and sodium alginate 8%). The flux coefficient values increased (Table 3), suggesting that permeation of aceclofenac increased with the addition of propylene glycol. It is believed that increasing the concentration of an active compound (aceclofenac) with in the solvent (propylene glycol) can give rise to persistent solvated complexes and evidence for that higher permeation of drug. In general, it is beveled that increase in the concentration of propylene glycol in gels, increases permeation of drug. However, the flux and permeability coefficient values decreased, comparatively as the concentration of propylene glycol increased (from 5 to 10%). Trottet et al 2004 [32] studied the permeation of drug (loperamide) linked with propylene glycol dose loading. They found a two or three order of magnitude difference in flux in between these two parameters considered. Firstly, to be because of large difference in the size of propylene glycol and loperamide (molecular weight 76 and 447, respectively), and lipophilicity and solubility characteristics. Secondly, the differences in permeation profiles of the two molecules, where propylene glycol found to cross the skin barrier more quickly than loperamide. A large molecule more likely to be delayed compared to a smaller one because of specific binding or interaction with the different element of the stratum corneum. The results of our study may be due to the higher dilution of aceclofenac in higher concentration of propylene glycol (10%) and higher flux of propylene glycol due to the lower molecular weight (while, the molecular weight of aceclofenac is 354.2). The addition of propylene glycol and/or increase in the concentration of the propylene glycol increased the bulk viscosity of the gels in order of. However, flux (permeability and drug release) values (Table 3) increased. This apparent lack of correlation between bulk viscosities of the gels and the flux (permeability and drug release) values indicate that the bulk viscosity is not the primary factor [26,33] that affect the release of aceclofenac. Among the formulations containing propylene glycol (5% to 10%), only formulations containing 5% propylene glycol showed higher flux (permeability and drug release) values. Among all the gel formulations, carbopol showed superior drug release than followed by Na CMC, HPMC and sodium alginate. The results shows that carbopol was good gelling agent as compare to other gelling agents for preparations of gel formulations. |
| |
Conclusion
|
| |
| From above results, we can conclude that aceclofenac gel formulations prepared with different gelling agents: carbopol, HPMC, Na CMC and sodium alginate showed acceptable physical properties and drug release study. All prepared gel showed acceptable physical properties concerning color, homogeneity, consistency, spreadability and pH value. Among all gel formulations, carbopol gels shows superior drug release after that Na CMC, HPMC and sodium alginate shows decreasing order of drug release. In carbopol gel formulations, the drug release was decrease with increase in carbopol concentration because polymer concentration increases, viscosity increases. Viscosity is negatively related to the release of active substance (aceclofenac) from formulations. All gel formulations containing penetration enhancer (propylene glycol) were used at different concentrations (5 to 10%), among them formulations containing 5% propylene glycol showed higher flux (permeability and drug release) values. Stability studies in all gel formulations showed that, the physical appearance, drug content, pH, rheological properties, and drug release in all gel formulations remain unchanged upon storage for two months. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure A |
Figure B |
Figure C |
Figure D |
|
| |
| |
| |










