Keywords
Mouth dissolving film, Gelatin, Solvent casting method.
Introduction
Route of drug administration is one of the common problems associated with the elderly and pediatric patients, including dysphasia and difficulty in swallowing of tablet and capsule in many medical conditions, like stroke, Parkinson’s, AIDS, thyroidectomy, head and neck radiation therapy and neurological disorders [1]. Fast dissolving films recently have acquired great importance in the pharmaceutical industry due to their unique properties and specific advantages like no need of water for disintegration, accurate dosing, and rapid onset of action, ease of transportability, ease of handling, pleasant taste and improved patient compliance [2].
Film delivery system consist of a very thin oral strip, which is simply placed on the patient’s tongue, instantly film is wetted by saliva; then film rapidly dissolves releasing the medication; this results in quick onset of action [3, 4].
It can also improved solubility, stability, biological half life and bioavailability enhancement of drug [5].
Zolpidem is preferentially use for the short term treatment of insomnia. It comes under Class I drug in BCS classification system, pKa value is 6.2, onset of action is within 15 minutes, half life is 2-3 hours, rate of absorption is more in empty stomach and have 70% oral bioavailability [6]. It has low solubility in water but is easily soluble in aqueous buffer solution (pH 6.8), HCL, and H2SO4 [7, 8]. Adult’s dose is 10 mg/day and for geriatric 5mg daily dose before meal [9].
An approach has been taken to formulate and evaluate the mouth dissolving films of Zolpidem tartrate for fast dissolution and absorption of drug, which will produce rapid onset of action and enhance oral bioavailability. Gelatin film forming polymer was used because it is a water soluble polymer, quickly dissolves in saliva and within seconds, drug is start to release from matrix [10].
Materials and Method
Materials
Zolpidem tartrate was received as gift samples. Gelatin, propylene glycol, Tween-80, Aspartame and menthol were purchased from Research lab Fine chem. Industries, Mumbai.
Method of preparation of mouth dissolving films Zolpidem tartrate
Gelatin in various concentrations was optimized as a primary film former and polyethylene glycol was used as a plasticizer. The composition formulas are given in Table 2. Distilled water was used as a vehicle for preparing various film compositions. Care was taken to avoid air entrapment. The film was casted by pouring the solution and spreading on a stainless steel plate. The film was air dried for 24 hours. The film was carefully removed from the plate, cut 2cm x 2cm size of film. Drug content was calculated as 5 mg per film of 4sq.cm.
Estimation of tartrate in bulk Drug [7]
Estimation of tartrate in drug was performed by the procedure give in USP.
Drug polymer compatibility study by FTIR [11]
Drug polymer compatibility studies were carried out by FTIR Spectrum study. The drug, individual excipients and mixture of drug and excipient were placed in the sample holding cavity of the instrument and the spectra were recorded by attinuated total reflectance on an FTIR spectrophotometer (Shimadzu IR Affinity -1).
Film Evaluation Parameters
1. Appearance
Film was checked visually for homogeneity, color, transparency.
2. Weight variation [13]
Weight variation was studied by randomly select the films from each batch and checked for average weight and individual sample weight. Variance in weight and SD was calculated.
3. Drug content [14]
A film of size 4 cm2 was cut and put into 10ml of volumetric flask which containing pH 6.8 phosphate buffer solution; shake well to get a homogenous solution and filter. Solution was suitably diluted and the absorbance was measured at 238 nm under UV Spectrophotometer, and then calculated percent drug content in film.
4. Thickness [12]
The thickness of the film was measured using digital Vernier Calliper at five different places of the film and average was taken and SD was calculated.
5. Folding endurance [15]
Folding endurance was determined by repeatedly folding the film at same place till it break. The number of times the film could be folded at the same place without breaking/cracking gives value of folding endurance. Average was taken and SD was calculated.
6. Residual solvents
In film, water or solvent may be present in residual form. Residual solvent was determined by taking initial weight (W1) of the film and then it is kept in hot air oven at 50°C for 20 min, let the film come to its normal temperature and then again weigh it. Calculate % loss on drying.
7. Surface pH [15]
The surface pH of films was determined in order to investigate the possibility of any side effect in vivo. An acidic or alkaline pH of film may cause irritation of the oral mucosa. It was determined to keep the surface pH as close to neutral as possible. The pH of films was measured by dissolving film in 10ml of distilled water; filter it, and measure the pH by pH meter. Average was taken and SD was calculated.
8. Dissolution Test [14]
Dissolution test was carried out using the standard basket apparatus described in USP type I. The dissolution was carried out in 400 ml of pH 6.8 phosphate buffer maintained at 37±0.50C at 50 rpm. 4 ml aliquots of samples were taken at 30 sec time intervals up to film will completely dissolve and maintained in sink condition with same volume of fresh pH 6.8 phosphate buffer. Aliquots samples were then diluted with phosphate buffer solution and drug release is determined spectrophotometrically at λmax 238 nm.
Results and Discussion
Estimation of tartrate
The result of test resembles the criteria given in pharmacopeia; solution of sample was giving dark blue colour. This confirmed the Zolpidem is present in given drug sample.
Drug polymer compatibility study by FTIR
The spectrum peaks of drug and excipients mixture that obtain were matched with the pure drug and individual peaks of excipients, and hence it was shown that polymer and excipients were compatible with drug, no any interaction was found. The spectrum of Zolpidem reveals the presence of major functional group in the structure of Zolpidem and peaks were supporting its identity. Similar peaks were observed in mixture sample.
| Wave Number (cm-1) |
Corresponding Functional Group and Type of Molecular Vibration |
| 823.6 |
Aromatic C-H (B) |
| 1506.41 |
Aromatic C=C (B) |
| 1633.71 |
Alkenyl C=C |
| 1654.92 |
Amide C=O |
| 2358.94 |
Amide N-H (B) |
Table No 1: Peaks observed in Infrared Spectrum Study
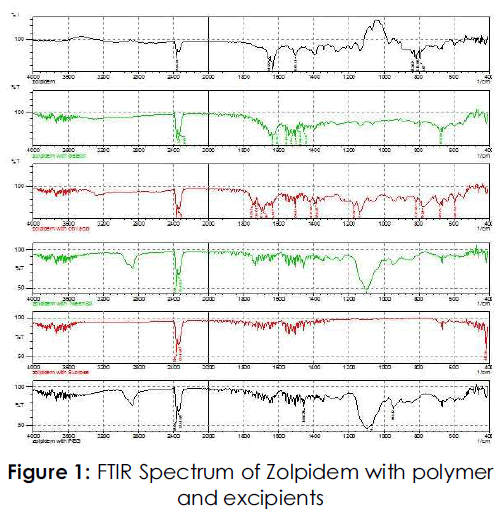
Figure 1: FTIR Spectrum of Zolpidem with polymer and excipients
Figure 2: Mouth dissolving films of Zolpidem tartrate
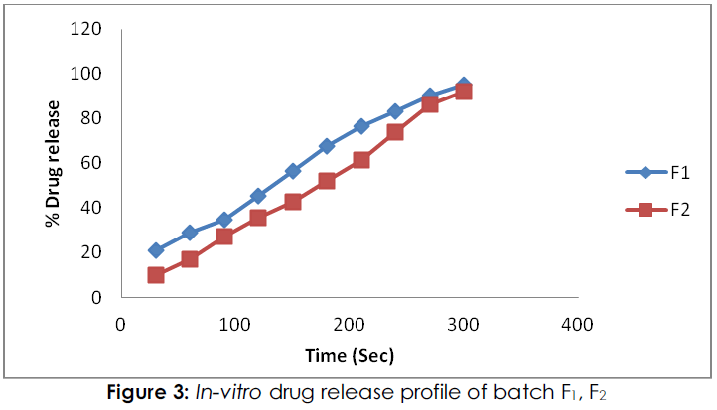
Figure 3: In-vitro drug release profile of batch F1, F2
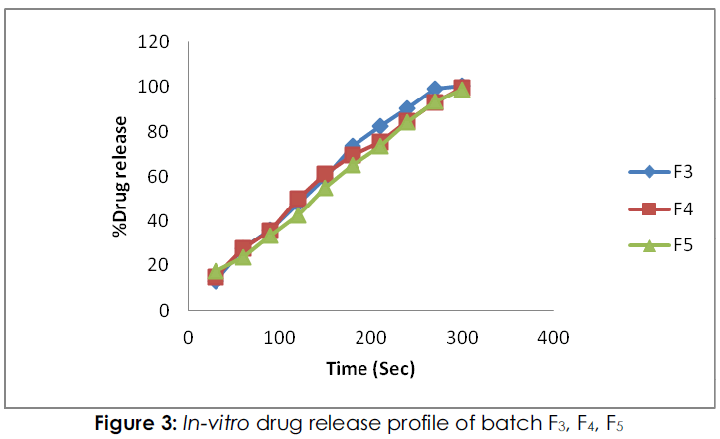
Figure 3: In-vitro drug release profile of batch F3, F4, F5
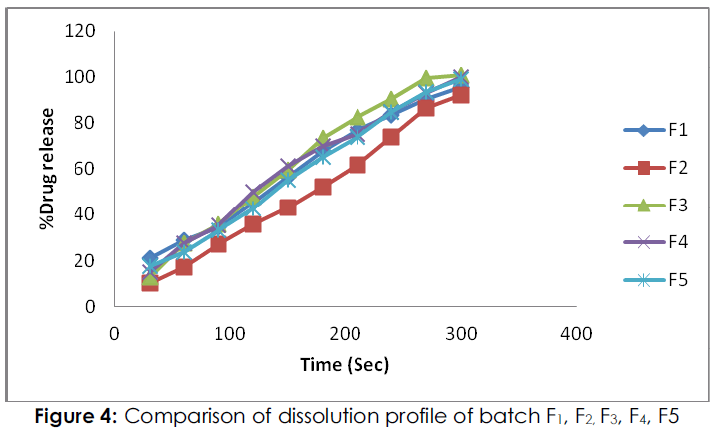
Figure 4: Comparison of dissolution profile of batch F1, F2, F3, F4, F5
Optimization of formulation variables
a. Study of mechanical properties
To study of mechanical property of film; optimization of PEG-400 concentration in film were evaluated. In batch F1 and F2; respectively 2% and 1% concentration of PEG was used. Films of F2 batch were exhibited high tensile strength, hard, low elastic modulus and unacceptable percentage of drug release because of concentration of PEG is low and in film; property of polymer was more intent. In F1; 2% PEG was used, film has moderate tensile strength, acceptable flexibility, softness, and satisfactory mechanical strength. There for in further batches same concentration of PEG was used. F3 batch was found be exhibited good mechanical properties.
b. Palatability of films
Various composition formulation batches were prepared to optimized the palatable taste of films. Batch F1 were formulated without flavours and citric acid; the taste of films were unacceptable. Batch F4 and F5 were formulate to optimize the combination of sweeteners and citric acid. Aspartame was used in F4 batch; only aspartame with of citric acid could not sufficient for taste masking of drug and if increase the concentration it; aspartame was itself produced a bitter taste. In batch F5 sucrose was used; but sucrose was not improved the palatability of drug, effect of citric acid was more intent and film not have suitable taste.
Therefore, in Batch F2, and F3 included in further study, they were contained aspartame, sucrose, citric acid and flavours. Batch F2 were improve the palatability of drug because of combination of taste masking agents; but this films found to be stiff. In F3 batch complete taste masking was achieved; concentration of citric acid and sucrose nullifies the bitter taste of zolpidem. Hence F3 formulation was suitable for formulation of mouth dissolving film of zolpidem by gelatin polymer. This batch was used for the further validation study and result of evaluation parameter of selected formulation was show in tables.
Physic-chemical properties of films
All the prepared formulation films were observed semi transparent with uniformly colour distribution. The values of weight variation were in ranged of 46 to 54 mg. The thickness of films were observed (<1 mm), very thin film. The variation in weight and thickness were might be due to variation in quantity of ingredients. The pH of the films was found in range of 6.842 to 6.960, which means that pH of films was found to be near around the saliva pH. Difference in the pH-value might be due to polymer used for film forming as well as the drug. The folding endurance was measured manually, by folding the film repeatedly at a point till it broke. The value of it was found less than 260; hence films have good physical and mechanical properties.
All film formulation was assayed for drug content. Uniformity of drug content among the batches was observed 99%. The results indicate that the process employed to prepare mouth dissolving film was worked and produce uniform film with minimal content variations.
Dissolution Test
The in-vitro dissolution studies was performed using 400 ml of phosphate buffer (pH 6.8) and was maintained at 37 ± 0.5ºC. The results of test were given in the Table No. 5.
From the in-vitro dissolution study; percent drug release in formulation F1, F2 were 95.368 %, and 92.146% respectively within 5 minutes while the formulation F3, F4 and F5 showed the drug release of 100.546%, 99.68 % and 98.880 % respectively within 5 minutes. The percent drug release in batch F2 low because films were exhibited high tensile strength, and hard, due to this it was required more time for dissolution. Form entire formulation; batch F3 was shown maximum drug release.
| |
Formulation code |
| Composition |
F1 |
F2 |
F3 |
F4 |
F5 |
| Drug |
50 mg |
50 mg |
50 mg |
50 mg |
50 mg |
| Gelatin |
100 mg |
100 mg |
100 mg |
100 mg |
100 mg |
| PEG 400 |
225.2
mg |
112.6
mg |
225.2
mg |
225.2
mg |
225.2
mg |
| Aspartame |
80 mg |
80 mg |
80 mg |
100 mg |
- |
| Sugar |
300 mg |
300 mg |
280 mg |
- |
300 mg |
| Citric acid |
- |
70 mg |
50 mg |
70 |
70 mg |
| Tween- 80 |
110.5
mg |
110.5
mg |
110.5
mg |
110.5
mg |
110.5
mg |
| Colour, flavours |
- |
q.s |
q.s |
q.s |
q.s |
| Water |
q.s |
q.s |
q.s |
q.s |
q.s |
Table 2: Composition of Zolpidem Tartrate mouth dissolving films
| Formulation code |
Weight variation (mg) |
Thickness(mm) |
Folding endurance (no. of folds) |
| F1 |
53.398 ± 0.398 |
0.871 ± 0.069 |
249.7 ± 4.271 |
| F2 |
46.642 ± 0.503 |
0.988± 0.060 |
260.0 ± 3.366 |
| F3 |
51.237 ± 0.267 |
0.814 ± 0.035 |
256.5 ± 3.415 |
| F4 |
53.451 ± 0.533 |
0.661 ± 0.076 |
252.5 ± 3.109 |
| F5 |
53.584 ± 0.615 |
0.916 ± 0.077 |
255.5 ± 1.290 |
All values are mean of 4 readings ± standard deviation
Table 3: Evaluation of weight variation, thickness, and folding endurance of mouth dissolving films of Zolpidem tartrate
| Formulation code |
Drug content (%) |
Residual solvents (%) |
Surface pH |
| F1 |
98.657± 0.286 |
4.092 ± 0.842 |
6.842 ± 0.047 |
| F2 |
99.602 ± 0.638 |
4.290 ± 0.347 |
6.890 ± 0.085 |
| F3 |
99.102 ± 0.286 |
3.450 ± 0.974 |
6.905 ± 0.111 |
| F4 |
99.88 ± 0.405 |
2.148 ± 0.517 |
6.930 ± 0.086 |
| F5 |
100.213 ± 0.462 |
3.605 ± 0.776 |
6.960 ± 0.104 |
All values are mean of 4 readings ± standard deviation
Table 4: Evaluation of drug content, residual solvent, surface pH of mouth dissolving film of Zolpidem tartrate
| Time (sec) |
Formulation Code |
| F1 |
F2 |
F3 |
F4 |
F5 |
| 30 |
20.967 |
10.013 |
12.857 |
14.857 |
17.368 |
| 60 |
29.024 |
17.254 |
27.635 |
27.657 |
23.647 |
| 90 |
34.631 |
27.191 |
35.857 |
35.502 |
33.202 |
| 120 |
45.251 |
35.791 |
47.991 |
50.035 |
42.635 |
| 150 |
56.457 |
42.879 |
59.324 |
61.146 |
55.013 |
| 180 |
67.691 |
52.235 |
73.557 |
69.727 |
65.213 |
| 210 |
76.782 |
61.480 |
82.457 |
75.213 |
73.746 |
| 240 |
83.257 |
73.813 |
90.257 |
84.746 |
84.391 |
| 270 |
90.364 |
86.217 |
99.102 |
92.946 |
93.435 |
| 300 |
95.368 |
92.146 |
100.546 |
99.68 |
98.880 |
Table 5: Comparative in vitro dissolution of formulation in pH 6.8 phosphate buffer
Conclusion
From the results of present work instimate, it was concluded that mouth dissolving film of Zolpidem tartrate were prepared by using 1% solution of gelatin as a film former. The flavours and sweeteners were masked the bitter taste of drug. Form the formulation study it was concluded that batch F3 has all the required specification; uniform drug content, excellent physical properties and less dissolution time. Hence, films of gelatin were suitable for the immediate drug delivery of Zolpidem tartrate, for the quick drug release and also it enhance the bioavailability of drug
7283
References
- Desu P.K, Sahu M. Formulation and Evaluation of Fast Dissolving Films of Zolmitriptan. IRJP 2012; 3(5):373-376.
- Bhyan B, Jangra S. Formulation and evaluation of fast dissolving sublingual films of Rizatripton Benzoate. Int.J.Drug Dev. &Res. 2012; 4(1):133- 143.
- Kaur R, Bala R. Exploration of different polymers and optimization of concentration of plasticizer in the formulation of oral fast dissolving strips. IJPRBS 2012; 1(2):94-101.
- Arya A, Chandra A, Sharma V, Pathak K. Fast Dissolving Oral Films: An Innovative Drug Delivery System and Dosage Form. Int.J.ChemTechRes. 2010; 2 (1):576-583.
- Kunte S, Tandale P. Fast dissolving strips: A noval approach for the delivery of verapamil. J Pharm Bioallied Sci. 2010; 2(4):325-328.
- Tripathi KD. Essential of medical Pharmacology, ed 6, New Delhi, Joypee brother’s medical publishers, 2006, pp 398.
- United States of Pharmacopeia 34, NF 29, Vol 3. Rockville: the United States Pharmacopeial convention, 2011, pp 4635.
- Mathrusri Annapurna, B. Swathi, M Siri Chandra, K Tulsi. Derivative spectrophotometric methods for the determination of zolpidem tartrate in tablets. JAPS 2012; 2(11):096-099.
- Mahajan M, Sawant S. Validation spectrophotometric method for the estimation of zolpidem tartrate in bulk and tablet formulation. Int.J. ChemTech Re. 2012; 4(1):403-408.
- Nagar P, Chauhan I, Yasir M. Insights into Polymers: Film Formers in Mouth Dissolving Films. Drug Invention Today 2011; 3(12):280-289.
- Mishra R and Amin A. Formulation and characterization of rapidly dissolving films of cetirizine hydrochloride using Pullulan as a film forming agent. Ind J Pharm Edu Res 2011; 45(1):71-77.
- Mahajan A. Formulation and evaluation of fast dissolving buccal films of sertraline. Int.J.Drug. & Res. 2012; 4(1):220-226.
- Senthikumaran, Jeganath S. Design, preparation and characterization of oral disintegrating films of candesartan cilexetil. IJRPNS 2012; 1(2): 269- 273.
- Ghorwade V, Patil A, Hullale A. Fast dissolving films: A novel approach for the delivery of montelukast sodium. Int J Pharm Sci. 2011; 4(2):228-232.
- Prabhakara P, Malli R, Koland M, Vijaynarayana K, D’Souza U, Harish NM, Shastry CS, Charyulu RN. Formulation and evaluation of fast dissolving films of levocitirizine di hydrochloride. Int J Pharm Investig 2011; 1(2): 99-104.











