Key words
|
| |
| salbutamol sulphate, bronchial asthma, buccoadhesive compacts |
| |
Introduction
|
| |
| Bronchial asthma is defined as a disease characterized by increased responsiveness of the bronchi to various stimuli manifested by widespread narrowing of the airways that reverses either spontaneously or as a result of treatment. Asthma is one of the common disease affecting the human race, 2-10% of the adult population suffer from the asthma or symptoms of asthma. Asthma has a diurnal rhythm, in large percent of patients the pulmonary function is reduced from midnight until 8 hours. Thus the ideal therapeutic agent should be effective at preventing branchospasm for 6-8 hours, during which most individuals are asleep. A limiting factor is the relatively short duration of bronchodilator activity of β2-adrenergic agonists. Adrenaline and Isoprinosine are degraded rapidly and thus cannot produce the desired duration of action. The synthetic derivatives Salbutamol, Terbutaline and Orcipranaline although less rapidly degraded, cannot be given in sufficient quantity to provide the desired duration of effect. Thus sustained release dosage forms such as buccoadhesive compacts, matrix tablets, patches, films are suitable for antiasthamatic therapy1. Salbutamol sulphate is β2-adrenergic agonists. It reverses branchospasm and reduces airways resistance. Salbutamol sulphate causes dilatation of large airways as shown by increased specific airways conductance and forced expiratory volume. It also improves small airways functioning as reflected in FEF 25 and FEF 75 of vital capacity. Salbutamol is available in market as tablet, pressurized aerosol, rotocaps for inhalation, nebulizer solution and syrup2. Salbutamol suphate is given orally 6-16mg in devided doses: slow I.V. injection, equivalent of 250µg of salbutamol: by I.V. infusion, the equivalent of 3-20µg of salbutamol/min3 |
| |
| Buccoadhesive delivery system has several advantages over conventional dosage forms. It significantly reduces dose, maintains constant blood levels for longer time, offers greater bioavailability and avoids first pass metabolism and large fractions of the drug goes in to systemic circulation4. |
| |
| Hence in the present work is to design and develop controlled release buccoadhesive compacts of salbutamol sulphate using carbopol 934P, carbpol 974P and Hydroxy prophyl methyl cellulose. |
| |
MATERIALS AND METHODS
|
| |
| Salbutamol sulphate, Carbopol 934P, HPMC- 4KM, gift sample obtained by BPRL & Kemwell Pvt Ltd-Bangalore, Carbopol 974P gift sample obtained by Dr. Reddy's Pvt Ltd-Hydarabad. Lactose, Magnesium Stearate, Sodium hydroxide, Potassium Di hydrogen ortho phosphate, Sodium chloride, Potassium chloride, Magnesium chloride, Sodium bicarbonate, Sodium di hydrogen ortho phosphate, Glucose, Sodium alginate and calcium chloride-Analytical grade. The reagents required for the present experimental work are Phosphate buffer pH 6.65, Tyrode solution6 and 1% (W/V) colloidal solution of sodium alginate solution7. |
| |
|
Preparation of buccoadhesive compacts of Salbutamol sulphate
|
| |
| Buccoadhesive compacts were prepared by direct compression method. All the ingredients were passed through #100 and were blended in mortar for uniform mixing. Blending was done separately for core, peripheral and backing layers. The blended powder of core layer was compressed using 7mm flat faced tablet punches. The core layer was then removed, placed in the center of a 10mm die cavity filled with ingredients of peripheral layer and was compressed. Then the upper punch was raised and ingredients of backing layer was added and finally compressed to a pressure of 14 units. |
| |
| In the present work, 16 formulations (F1 to F16) Buccoadhesive compacts of Salbutamol sulphate in two different concentrations (4mg and 8mg / compact) were prepared using variable concentrations of carbopol- HPMC 4KM(1:0, 1:1, 1:2 and 0:1). |
| |
EVALUATION
|
| |
|
a. Weight uniformity studies
|
| |
| The weights of each of 10 individual BCs were determined by weighing on an electronic balance. The weight data from the BCs were analyzed for sample mean and standard deviation. |
| |
|
b. Hardness uniformity studies
|
| |
| The hardness of BCs was measured by using Pfizer hardness tester. Five BCs were used for hardness uniformity studies. The hardness data used to calculate mean and standard deviation. |
| |
|
c. Thickness uniformity studies
|
| |
| The thickness uniformity studies were carried out by using Vernier caliperse. Five BCs were used for thickness uniformity studies and denoted in millimeter. The data obtained was used to calculate mean and standard deviation. |
| |
|
d. Content uniformity studies
|
| |
| 10 BCs were accurately weighed and they were powdered in mortar using pestle until uniform powder is obtained. The weight equivalent to 4mg or 8mg was weighed and transferred to 50ml volumetric flask. The volumetric flask was shaken for 15 min. Small quantity was transferred to centrifuge tube and centrifuged for 5 min at 200rpm. Then the solution was filtered and the absorbance was measured at 276nm. |
| |
|
e. in vitro bioadhesion studies
|
| |
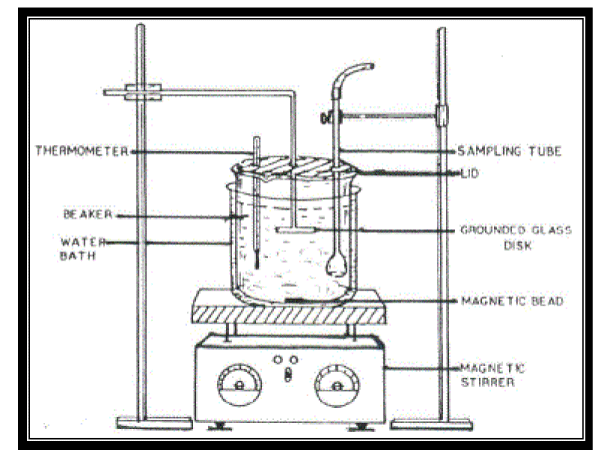
| The apparatus used for in vitro bioadhesion studies is shown in Figure No. 02. in vitro bioadhesion studies were carried out using pig buccal mucosa and modified two armed balance. The beaker on one side of the balance was counter balanced by using suitable weights on the other side. The BC was fixed to the tissue holder with cyanoacrylate adhesive. A circular piece of pig buccal mucosa was fixed to the tissue holder with cynoacrylate adhesive and was immersed in tyrode solution and the temperature was maintained at 37±1°C. Then the BC was placed on the buccal mucosa by using a preload of 50gms and kept it aside for 5 min to facilitate adhesion bonding. After preloading time, the preload was removed and the water was allowed to flow into the beaker kept on the other side of the balance at the flow rate of 1 drop/sec until the BC detaches from the buccal mucosa. The weight required to detach the BC from the buccal mucosa was noted8. Similarly in vitro biadhesion studies was also carried out using 1 %( W/V) colloidal solution of sodium alginate as a model mucosal membrane. The weight required to detach the BC from the buccal mucosa was noted7. |
| |
|
f. in vitro dissolution studies
|
| |
| in vitro dissolution studies were carried out using modified dissolution apparatus. The modified dissolution apparatus is shown in Figure No. 02. The dissolution studies were carried out using 500ml of phosphate buffer pH 6.6. The BC was fixed to the ground glass plate immersed in phosphate buffer solution. The temperature was maintained at 37±1°C and stirred at 100 rpm on a temperature controlled hot plate with a magnetic stirrer. Aliquots were withdrawn at regular intervals of time up to 8 hours with the help of guarded pipette and same volume was replaced with phosphate buffer pH 6.6. The absorbances of these solutions were measured at λmax 276nm8. |
| |
| The drug availability is usually determined by the rate of its release from the dosage form. Dissolution is the best available tool today that can reveal at least quantitatively some information about the physiochemical availability of the drug. It helps in comparing the consistency of drug availability within the same formulation and between different formulations. The release of drug from majority of oral sustained release system occurs by diffusion, dissolution or a combination of both. |
| |
| The most widely accepted theory of dissolution is the film theory or diffusion layer model. According to this theory, dissolution involves two consecutive steps, first solution of the solid to forms a thin film or layer at the solid/liquid interface, and Secondly the diffusion of soluble solute from stagnant layer to the bulk of solution. |
| |
|
g. Swelling Index
|
| |
| Four BCs were weighed individually (designated as W1) and placed separately in Petri dishes containing 4ml of phosphate buffer pH 6.6. The petri dishes were kept at room temperature. At regular intervals i.e. 0.5, 1.0, 2.0 and 4.0 hours the BCs were removed from petri dishes and excess water was removed carefully by using filter paper. The swollen BCs were weighed (W2). The swelling index was calculated by using the formula |
| |
| Swelling Index = (W2-W1)/W1 |
| |
|
h. Fourier Transformed Infrared (FT-IR) spectroscopy
|
| |
| The sample powder was dispersed in KBr powder and analyzed. FT-IR spectra were obtained by powder diffuse reflectance on a spectrophotometer type FT-IR 1600 Perkin Elmer Co., Yokohama, Japan. |
| |
RESULTS AND DISCUSSION
|
| |
|
Content uniformity
|
| |
| The results obtained showed that the drug content of formulation were within the I.P.limits. The results of content uniformity of salbutamol sulphate BCs are given in Table No. 02. |
| |
|
Physical properties
|
| |
| The results showed that weight variation, thickness were lying within limits. Because of variation in the compressional forces there is a slight variation in hardness of BCs. The results of Physical properties of salbutamol sulphate BCs are given in Table No. 02. |
| |
|
in vitro Bioadhesive Strength Determination Studies
|
| |
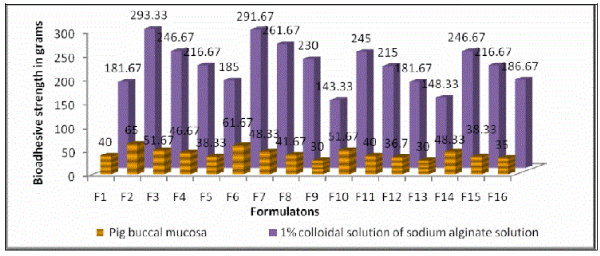
| in vitro bioadhesion studies were carried out using bioadhesion apparatus with pig buccal mucosa. The results of bioadhesive strengths of salbutamol sulphate BCs are given in Table No. 02. and are graphically represented in Figure No. 03. The bioadhesive strength of salbutamol suphate (4mg) BCs containing carbopol 934P is in the following order, F2>F3>F4>F1. The bioadhesive strength of salbutamol sulphate (8mg) BCs containing carbopol 934P is in the following order, F6>F7>F8>F5. The bioadhesive strength of salbutamol sulphate (4mg) BCs containing carbopol 974P is in the following order, F10>F11>F12>F9. The bioadhesive strength of salbutamol sulphate (8mg) BCs containing carbopol 974P is in the following order, F14>F15>F16>F13. The maximum bioadhesive strength was observed in BCs containing Carbopols and HPMC in the ratio 1:1 followed by 1:2, 0:1 and 1:0. It was also seen that the bioadhesive strength did not changed significantly altered as the drug concentration increased from 4mg to 8mg/BC. |
| |
| BCs with Carbopol 974P showed lesser bioadhesive strength when compared to BC of corbopol 934P. |
| |
| Similarly in vitro bioadhesion studies were also carried out using 1 %( W/V) colloidal solution of sodium alginate as a model mucosal membrane. The results obtained are in the similar order, but exhibited lesser bioadhesive strength when the pig buccal mucosa was used. The results are in Table No. 02.and graphically represented in Figure No. 03. |
| |
|
in vitro dissolution studies
|
| |
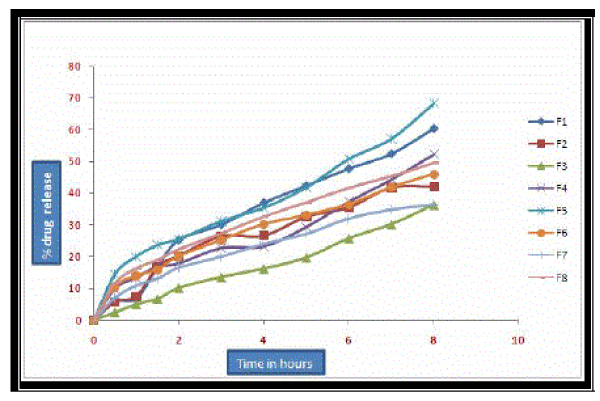
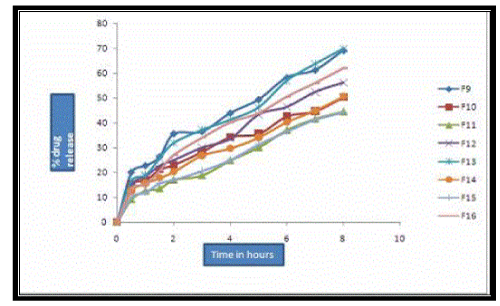
| In-vitro dissolution studies were carried out using modified dissolution apparatus using the dissolution media phosphate buffer pH 6.6 for 8 hours. From the dissolution studies it was observed that the BCs of Salbutamol sulphate in different concentrations and Carbopol –HPMC in different ratios have shown a delayed release of the drug. The results of dissolution studies of salbutamol sulphate BCs are given in tables- 03 and graphically represented in four different models in Figure No. 04 & 05. |
| |
| The dissolution rate of Salbutamol sulphate (4mg) BCs containing Carbopol 934P and HPMC in different concentrations was in the following order. F1>F4>F2>F3. The dissolution rate of Salbutamol sulphate (8mg) BCs containing Carbopol 934P and HPMC was in the following order, F5>F8>F6>F7. The dissolution rate of Salbutamol sulphate (4mg) BCs containing Carbopol 974P and HPMC in different concentrations was in the following order. F9>F12>F10>F11. The dissolution rate of Salbutamol sulphate (8mg) BCs containing Carbopol 974P and HPMC was in the following order, F13>F16>F14>F15. The results obtained indicated that the rate of drug release was found to be more in F1, F5, F9 and F13 which was due to rapid ionization of CP-934P and CP- 974P. As the concentration of HPMC 4KM increases the rate of drug release is decreased (F2, F3, F6, F7, F10, F11, F14 & F15). However formulation which contains only HPMC 4KM, exhibited less drug release compared to formulations containing carbopol. This may be attributed to the stability of HPMC polymer over a wide range of acid and alkaline conditions. |
| |
|
Swelling Studies
|
| |
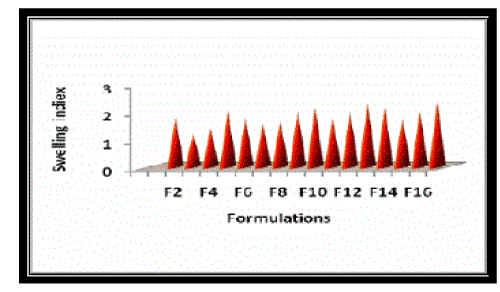
| The swelling studies of BCs of Salbutamol sulphate were conducted using phosphate buffer pH 6.6 in petri dishes. It was carried out for 4 hours. The liquid uptake by the BCs was calculated at different time intervals and are reported in Table No. 04. The swelling index of BCs of Salbutamol sulphate was plotted with swelling time profile as the function of time and is given in Figure No. 06. |
| |
| The swelling index of Salbutamol sulphate (4mg) BCs containing carbopol 934P showed swelling rates in the order, F4 > F1 > F3 > F2. The swelling index of Salbutamol sulphate (8mg) BCs containing carbopol 934P showed swelling rates in the order, F8 > F5 > F7 > F6. The swelling index of Salbutamol sulphate (4mg) BCs containing carbopol 974P showed swelling rates in the order, F12 > F9 > F11 > F10. The swelling index of Salbutamol sulphate (8mg) BCs containing carbopol 934P showed swelling rates in the order, F16 > F13 > F15 > F14. It was observed that the swelling rate increased with an increase in HPMC 4 KM content of the compacts, except formulation F1, which contained carbopol only and initially showed less swelling than other formulations. |
| |
| The BCs containing Carbopol 974P showed higher swelling index values than Carbopol 934P |
| |
Conclusion
|
| |
| Buccoadhesive compacts(BC’s) of Salbutamol sulphate in different concentrations (4mg and 8mg) were prepared by direct compression method using polymers like carbopols and hydroxyl propyl methyl cellulose 4KM(HPMC 4KM) in the ratios of 1:0, 1:1, 1:2 and 0:1. Also two different carbopols i.e. carbopol 934P (CP-934P) and carbopol 974P (CP-974P) varying in their molecular weight were used. The compacts were evaluated for weight uniformity, drug content uniformity, swelling index, in vitro bioadhesive strength and in vitro dissolution studies. Maximum bioadhesive strength was observed in compacts formulated with a combination of carbopol-HPMC 4KM (1:1). Formulations containing CP-934P exhibited higher bioadhesive strength as compared with CP-974P. Swelling was increased with an increase in HPMC 4KM contents in compacts. The in vitro dissolution studies were carried out using modified dissolution apparatus in phosphate buffer pH 6.6 for 8 hours. Formulations containing higher concentration of drug (8mg/BC) showed a slight higher rate of drug release as compared to the one with lower drug concentration (4mg/BC). The release rate was found to follow first order rate kinetics. The data were evaluated by a simple power equation (Mt/M∞=ktn), it was observed that all the compacts followed non- Fickian release kinetics. From FT-IR spectra it may be concluded that there is no chemical interaction between the drug and polymer. From the results of the present experimental work it may be concluded that buccoadhesive compacts of Salbutamol sulphate can be developed as potential prolonged release formulation with improved dissolution rate, which otherwise undergoes extensive first pass metabolism when administered orally. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
 |
 |
 |
| Figure 4 |
Figure 5 |
Figure 6 |
|
| |












