Keywords
Clarithromycin; Pullulan acetate; 2FI model; Formulation; Sustained release
Introduction
Helicobacter pylori are spiral-shaped bacteria that grow in the digestive tract and have a tendency to attack the stomach lining.H. pyloriinfections are usually harmless, but they are responsible for the majority of ulcers in the stomach and small intestine. It is associated with the development of serious gastro duodenal disease including peptic ulcers, gastric lymphoma and acute chronic gastritis [1-3]. This is because of the low concentration of the antibiotic reaching the bacteria under the mucosa, instability of the drug in the low pH of gastric fluid and short residence time of the antibiotic in the stomach. Triple therapy for treatment of H. pylori includes Proton pump inhibitor, Clarithromycin (500 mg), and Metronidazole (400 mg) or Amoxicillin (1 g) twice a day. Clarithromycin is semisynthetic macrolide antibiotic derived from erythromycin which is active against a variety of microorganisms. It is effective against Mycobacterium avium complex (MAC) and is used for the treatment of H. pylori-associated peptic ulcer disease [4-6]. One way to improve the efficacy in eradicating the infection is to deliver the antibiotic locally in the stomach. Better stability and longer residence time will allow more of the antibiotic to penetrate through the gastric mucus layer to act on H. pylori[7-9]. Pullulan is a good water soluble neutral linear polysaccharide consisting of α-1,6 linked maltotriose residues. It is broadly used as a food additive and is considered to be safe for human use. In present days material scientist are paying more and more attention to process the inorganiccrystallization studies within a largely formulated organic matrix of biologically synthesised compounds like Pullulan. They are generally used for various types of in-vitro and in-vivo drug delivery studies along with different types of therapeutic formulations etc. Pullulan is a hydrophilic polysaccharide by nature, so it is very essential to change the hydrophilic nature into a hydrophobic for its applications in the controlled drug delivery system which can be done by the substitution reaction (generally acetylation reaction). The best suitable acetylated-pullulan is Pullulan acetate, which was used for carrying the drug as polymers in previous studies done by various authors [10].
In the development of any pharmaceutical product for sustained release ability, an important issue is to design a formulation with optimized quality in a short time period and minimum number of trials [11-13]. The response surface methodology has been commonly used for designing and optimization of different pharmaceutical formulations, which requires minimum experimentation. Thus, it is less time-consuming and cost-effective than the conventional methods of formulating dosage forms. Based on the design of experiments, response surface methodology encompasses the generation of polynomial equations and of the response over the experimental domain to determine the optimum formulation [14,15].
The main objective of the current investigation was to develop Clarithromycin sustained release formulaion loaded with a hydrophobic polymer, pullulan acetate by using response surface methodology. A computer-aided optimization technique using factorial design (4-Factors, 2-Levels) was employed to investigate the eect of the amounts of various parameters namely, RPM, pH, Pullulan acetate concentration and Clarithromycin concentration in order to find optimum combination for maximum release of the Clarithromycin from the polymer.
Materials and Methods
Chemicals
The Clarithromycin (CLN) was purchased from Square Pharmaceuticals Ltd. and Dichloromethane was taken from Sigma Chemical Co. (USA). Formamide, Pyridine (Chemical grade) and acetic anhydride were purchased from Hi media, India. Pullulan was purchased from TCI Chemicals, India.
Synthesis of pullulan acetate from pullulan
The Pullulan acetate was synthesized from pullulan as per the standard method [16] given by Mishra and Suneetha. The synthesis of Pullulan acetates was done as follows; in which 2.5 g of pullulan was suspended in 25 ml of formamide solution and dissolved by vigorous stirring at 400 rpm. After that 60 ml of pyridine with 150 ml of acetic anhydride were added to this suspension which was stirred at 500 rpm for 2 days. The synthesized Pullulan acetate was extracted after reprecipitation from 250 ml of water.
Drug loading procedure
50 mg of pullulan acetate was made to dissolve in 5 mL of dichloromethane and to this solution 80 mg of Clarithromycin was added. Then, the solution was homogenised at gentle rpm in the room temperature until it was dissolved completely. In order to prepare the microspheres, this homogenised solution was dropped very slowly into 50 ml of double distilled water with stirred conditions in the homogeniser again to evaporate the organic solvent for 2 h at room temperature. Clarithromycin-loaded microspheres were obtained by centrifugation at 10,000 rpm, in 4°C for 15 min and then subjected to be dried at 70°C for 1 h to get the final microsphere samples. To get the amount of drug that had been loaded, the freeze-dried pullulan acetate microspheres were suspended in methanol, vigorously stirred for 30 min and then sonicated for 10 min. The resulting solution was centrifuged at 12,000 rpm for 20 min and the supernatant was taken for the measurement of drug concentration using UV spectrophotometer.
In-vitro release study
The release study of the drug loaded microspheres was carried out as follows; 50 mg of Clarithromycin-loaded microspheres in 1 ml of 0.15M phosphate buffered saline (PBS; pH 7.4) or HCI in PBS (pH 1.2) were put into two different dialysis tube which was introduced into 100 ml of respective buffer and was kept in a stirrer at gentle rpm at room temperature. At specific time intervals, the released sample was taken out to find the concentration of the released drug and replaced respectively with freshly prepared buffer. The concentration of the released drug was estimated by using UV spectrophotometer at 319 nm.
Experimental design for optimization
Different parameters like Time, RPM, Pullulan acetate concentration and Clarithromycin concentration were considered for the efficiency to release the loaded drug in the designed buffer. The whole analysis was done with Design Expert Software Version 7.0. In the Table 1 the low value and high value for the each factor has given. The design model was reduced 2FI model with 2 levels and 0 centre point. Total 8 runs were framed and each run was corresponded to different formulation such as F1, F2, F3, F4, F5, F6, F7 and F8 (Table 2).
| Factor |
Name |
Units |
Low Actual |
High Actual |
Mean |
Std. Dev. |
| A |
Pullulan Acetate |
mg/mL |
1.5 |
3.5 |
2.5 |
1 |
| B |
Time |
Minute |
10 |
30 |
20 |
10 |
| C |
RPM |
|
500 |
15000 |
7750 |
7250 |
| D |
Drug Concentration |
mg/mL |
0.5 |
1.5 |
1 |
0.5 |
Table 1: Design of various Factors for the Analysis.
| Formulations |
Run |
A:Pululan Acetate (mg/mL) |
B:Time
(Minute) |
C:RPM |
D:Clarithromycin Concentration (mg/mL) |
| F1 |
1 |
3.5 |
10 |
15000 |
0.5 |
| F2 |
2 |
3.5 |
30 |
15000 |
1.5 |
| F3 |
3 |
1.5 |
10 |
500 |
0.5 |
| F4 |
4 |
1.5 |
10 |
15000 |
1.5 |
| F5 |
5 |
1.5 |
30 |
15000 |
0.5 |
| F6 |
6 |
3.5 |
30 |
500 |
0.5 |
| F7 |
7 |
1.5 |
30 |
500 |
1.5 |
| F8 |
8 |
3.5 |
10 |
500 |
1.5 |
Table 2: Various combinations of the factors.
In-vitro release study
Various parameters were optimized by the Design Expert Software Version 7.0. In the Table 3 total 8 formulations (F1…..F8) and their corresponding drug, Clarithromycin release % has been summarized.
Time in
Hour |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
| 0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| 1 |
25 |
35 |
5 |
20 |
43 |
38 |
18 |
8 |
| 2 |
45 |
55 |
22 |
38 |
68 |
62 |
34 |
22 |
| 3 |
57 |
68 |
34 |
50 |
77 |
70 |
50 |
37 |
| 4 |
70 |
82 |
45 |
65 |
92 |
86 |
67 |
40 |
| 5 |
75 |
85 |
55 |
68 |
95 |
90 |
70 |
45 |
| 6 |
76 |
86 |
54 |
69 |
95 |
89 |
71 |
45 |
| 7 |
74 |
84 |
56 |
69 |
96 |
92 |
71 |
46 |
Table 3: Different formulations and the corresponding release profile (The values are the average of 3 different set of experiment and was made round off).
From the Table 3 and Figure 1, it was found that the F5 was the optimum formulation, in which maximum amount of the drug was released could be reached more than 90% from the polymeric material. Hence F5 was considered for further study in the statistical optimization.

Figure 1: Release profile of different formulations (The values are the average of 3 different set of experiment).
Statistical optimization
Various combinations of different levels of the factors were generated by the Design Expert statistical software and according to it the experiments were conducted. The drug release % was tabulated for respective trials (Table 4). From the experiment it was found that F5 was showing maximum release of the Clarithromycin from the polymeric material. As per the results obtained from the experiment, it was fitted to Reduced 2FI Model. In this model, one factor will not be counted for the analysis by the Design Expert Software. The model summary has been summarized in Table 5. A negative “Pred R-Squared” implies that the overall mean is a better predictor of response than the current model. In addition to that “Adeq Precision” measures the signal to noise ratio. A ratio greater than 4 is desirable. In this model the ratio of 4.971 indicates an adequate signal. There fore, this model can be used to navigate the design space.
| Formulations |
Run |
A:Pululan Acetate (mg/mL) |
B:Time
(Minute) |
C:RPM |
D:Clarithromycin Concentration (mg/mL) |
Drug Release
(%) |
| F1 |
1 |
3.5 |
10 |
15000 |
0.5 |
75 |
| F2 |
2 |
3.5 |
30 |
15000 |
1.5 |
85 |
| F3 |
3 |
1.5 |
10 |
500 |
0.5 |
55 |
| F4 |
4 |
1.5 |
10 |
15000 |
1.5 |
68 |
| F5 |
5 |
1.5 |
30 |
15000 |
0.5 |
95 |
| F6 |
6 |
3.5 |
30 |
500 |
0.5 |
90 |
| F7 |
7 |
1.5 |
30 |
500 |
1.5 |
70 |
| F8 |
8 |
3.5 |
10 |
500 |
1.5 |
45 |
Table 4: Design Summary of different factors with actual response.
| Std. Dev. |
11.00568 |
|
R-Squared |
0.82385 |
| Mean |
72.875 |
Adj R-Squared |
0.588984 |
| C.V. % |
15.10213 |
Pred R-Squared |
-0.25262 |
| PRESS |
2584 |
Adeq Precision |
4.970833 |
Table 5: Model summary.
In general, a model fits the data well if the differences between the observed values and the model’s predicted values are small and unbiased. In general, the higher the R-squared, the better the model fits the data. Here, R-squared value was found to be 82.38%. Therefore, this model can be used for the study.
ANOVA for selected factorial model
The “Model F-value” of 3.51 implies the model is not significant relative to the noise. There is 16.53% chance that a “Model F-value” this large could occur due to noise. Values of “Prob>F” less than 0.0500 indicate model terms are significant. In this case there are no significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve the model (Table 6).
| Source |
Sum of
Squares |
df |
Mean
Square |
F
Value |
p-value
Prob>F |
|
| Model |
1699.5 |
4 |
424.875 |
3.50774 |
0.1653 |
not significant |
| A-Pullulan Acetate |
6.125 |
1 |
6.125 |
0.050568 |
0.8365 |
|
| B-Time |
1176.125 |
1 |
1176.125 |
9.71001 |
0.0526 |
|
| C-RPM |
496.125 |
1 |
496.125 |
4.095975 |
0.1361 |
|
| AB |
21.125 |
1 |
21.125 |
0.174407 |
0.7043 |
|
| Residual |
363.375 |
3 |
121.125 |
|
|
|
| Cor Total |
2062.875 |
7 |
|
|
|
|
Table 6: ANOVA for the model terms.
Final equation in terms of coded factors
The different coefficient of the factors with standard error and digree of freedom have been summarized in the Table 7. The final equation in terms of codded factors has given below:
| Factor |
Coefficient
Estimate |
df |
Standard
Error |
95% CI
Low |
95% CI
High |
| Intercept |
72.875 |
1 |
3.891096 |
60.49179 |
85.25821 |
| A-Pullulan Acetate |
0.875 |
1 |
3.891096 |
-11.5082 |
13.25821 |
| B-Time |
12.125 |
1 |
3.891096 |
-0.25821 |
24.50821 |
| C-RPM |
7.875 |
1 |
3.891096 |
-4.50821 |
20.25821 |
| AB |
1.625 |
1 |
3.891096 |
-10.7582 |
14.00821 |
Table 7: Estimation of coefficient of different factors.
Drug Release (%)=72.875+0.875 × A+12.125 × B+7.875 × C+1.625× A × B
The final equation in terms of actual factors has given below:
Drug Release (%)=46.14439655-2.375 × Pullulan Acetate+0.80625 × Time+0.001086207 × RPM+0.1625 × Pullulan Acetate × Time
Interaction of different factors
Pullulan acetate and time: The interaction between Pullulan Acetate and Time was studied, in which it was found that when time of cross linking with the polymer increased, the drug release also increased. But, when Pullulan acetate concentration increased the drug released % was affected less as comparison to time (Figure 2a).
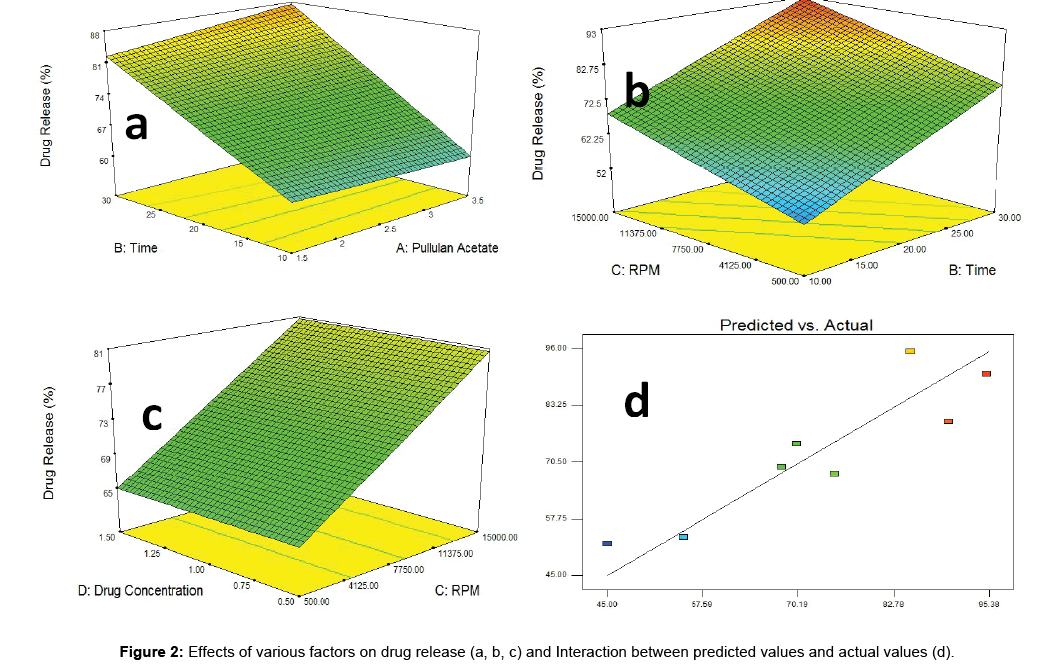
Figure 2: Effects of various factors on drug release (a, b, c) and Interaction between predicted values and actual values (d).
RPM and time: From the Figure 2b, it was found that, both RPM and Time has positive effect on the drug released. But time had more effect as comparison to RPM. Hence, the time of cross linking of drug Clarithromycin with pullulan acetate affects the % of drug release.
Drug concentration and RPM: From the Figure 2c and 2d it was found that RPM had more effect on the release than the drug concentration. When RPM increased, the drug released from the polymer was also increased. In addition to that, the drug loading concentration had no effect on the % of drug release.
Normal plot: All the factors having positive effect and negative effect for % of release of drug was analyzed through normal plot with Standardized effect on X-axis and Normality % Probability on Y-axis. In the Figure 3, it was found that factor A, B, C and AB having positive effect. In Shapiro-Wiki test, the W-value was found to be 0.947 and p-value was found to be 0.554. As all the points lied on the diagonal, the specified model was fitted nicely for this study.
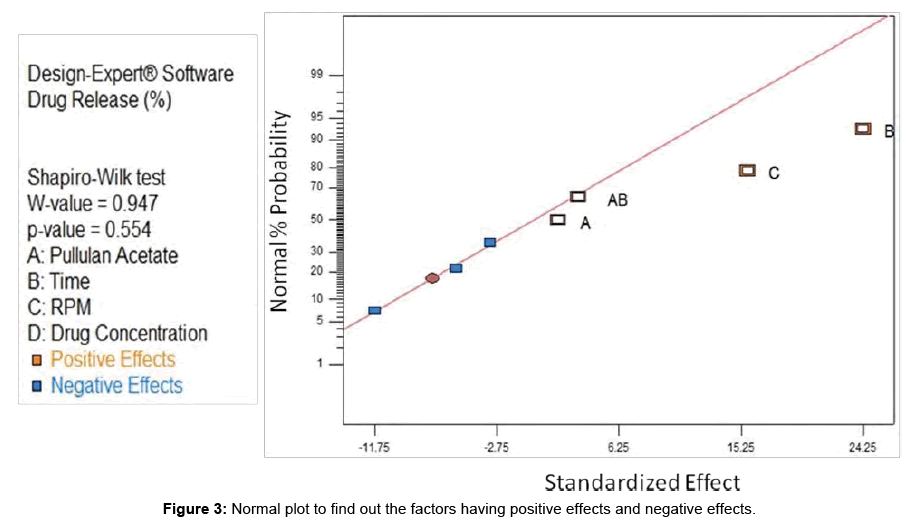
Figure 3: Normal plot to find out the factors having positive effects and negative effects.
Conclusions
In this study, we have tried to develop and optimize Claritromycin loaded with pullulan acetate for sustained release application by response surface methodology based on factorial design with reduced 2FI Model. Out of 8-formulaion, the 5th formulation (F5) was found to be optimum for the maximum release of the drug (more than 90%). This model was fitted with this study very nicely. From the 3-D plot, it was found that RPM, Time and Pullulan acetate concentration have positive effect on the release of the drug.
Acknowledgements
The authors want to acknowledge the financial support and laboratory facilities from Sreenidhi Institute of Science and Technology, Hyderabad to carry out this research work.
Conflict of Interest
The authors declare that they have no conflict of interest.
11112
References
- Yokel RA, Dickey KM, Goldberg AH (1995) Selective adherence of a sucralfate-tetracycline complex to gastric ulcers: Implications for the treatment of helicobacter pylori. Biopharm Drug Disposition 16: 475-479.
- Park SH, Chun MK, Choi HK (2004) Preparation of an extended-release matrix tablet using chitosan/Carbopolinterpolymer complex. Int J Pharm 347: 39-44.
- Patel JK, Bharadia PD, Amin AF, Patel MM (2004) Formulation optimization & evaluation of controlled release mucoadhesive microspheres of glipizide for oral drug delivery using factorial design. Drug Del Tech 4:48-53.
- Kubo W, Konno Y, Miyazaki S, Attwood D (2004) In situ-gelling pectin formulations for oral sustaineddelivery of paracetamol. Drug DevInd Pharm30:593-9.
- Zatz JL, Woodford DW (1987) Prolonged release of theophylline from aqueous suspension.Drug DevInd Pharm 13: 2159-2178.
- Ania AH, Gehanne AS, Nahed DM (2007) Preparation, in vitro and in vivo evaluation of stomach-specific Metronidazole-loaded alginate beads as local antiHelicobacter pylori therapy. J Control Release 119: 207-214.
- Tas C, Ozkan Y, Savaser A and Baykara T (2004) In-vitro and ex-vivo Permeation Studies of Chlorpheniramine Maleate Gels Prepared by Carbomer Derivatives. Drug DevInd Pharm30: 637-47.
- Shaji J, Lodha S (2008) Response surface methodology for the optimization of celecoxib self microemulsifying drug delivery system. Indian J Pharm Sci 70:585-90.
- Nazzal S, Smalyukh II, Lavrentovich OD, Khan MA (2002) Preparation and in vitro characterization of a eutectic based semisolid self-nanoemulsified drug delivery system (SNEDDS) of ubiquinone: Mechanism and progress of emulsion formation. Int J Pharm 235:247-65.
- Mishra B, Suneetha V, Kalyani R(2011) The role of microbial pullulan, a biopolymer in pharmaceutical approaches: A review. J Appl Pharm Sci 6:45-50.
- Shivkumar HN, Patel PB, Desai BG, Ashok P, Arulmozhi S (2007) Design and statistical optimization of gliclazide loaded liposphere using response surface methodology. Acta Pharm57:269-85.
- Singh I, Kumar P, Kumar S, Rana V (2010) Formulation and development of matrix tablets of tramadol using katira gum as release modiï¬ÂÂÂÃÂer. YakugakuZasshi 130: 1225-1231.
- Nayak AK, Pal D (2011) Development of pH-sensitive tamarind seed polysaccharide-alginate composite beads for controlled diclofenac sodium delivery using response surface methodology. Int J BiolMacromol49: 784-793.
- Varelas CG, Dixon DG, Steiner CA (1995) Zero-order release frombiphasic polymer hydrogels. J Control Release 34: 185-192.
- Shah M, Pathak K(2010) Development and statistical optimization of solid lipid nanoparticles of simvastatin by using 23 full-factorial design. AAPS Pharm Sci Tech 11: 489-496.
- Mishra B, Suneetha V(2012) Release study of Naproxen, A Modern drug from pH Sensitive Pullulan Acetate Microsphere.IntJ Drug Dev& Res 4: 259-262.









