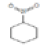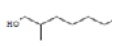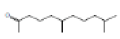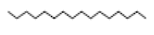Keywords
Gas chromatography-Mass spectrometry; Infra-red; Bioactive compounds; Therapeutic effect
Introduction
Plants are used as a source for many potent drugs [1], as plants synthesize lower molecular weight organic compounds which possess various biological activities [2-4]. A large number of medicinal plants and their purified constituents have shown therapeutic activities [5], and have proved as safe and effective therapeutic agents. Many plant species have been used in folklore medicine to treat various ailments [6]. The development of pharmaceuticals begins with identification of active principles, detailed biological assays and dosage formulations followed by clinical studies to establish safety, efficacy and pharmacokinetic profile of the new drug [7].
Combretum dolichopentalum is used traditionally for curing several ailments like stomach ache, gastro intestinal disorders (such as dysentery, passage of bloody stool and diarrhoea) and stomach ulcer in and around Ogwa in Imo State of Nigeria. Also in Ezinihitte Mbaise, Imo State, C. dolichopentalum is taken by women after parturition for reconditioning of the uterus [8]. Aqueous extracts of the leaves of C. dolichopentalum have antioxidant activities [9]. This study is a pioneer in identifying the organic compounds of C. dolichopentalum using GCMS analysis.
Materials and Methods
Sample collection and preparation
Combretum dolichopentalum belong to the family of Combretaceae. Fresh leaves of C. dolichopen9talum were harvested from a farm in Obinze in Owerri West Local Government Area of Imo State, Nigeria (N 5° 23’41.1’ and E 6° 57’ 14.0’). The sample specimen was deposited with voucher IMSUH12 at Imo State University herbarium. Fresh leaves of the plant were plucked from their stems, washed with distilled water and allowed to dry at room temperature (21-25°C). The dried samples were pulverized (using electric blender) and stored in an airtight container kept in a dark cupboard.
Preparation of samples for Gas Chromatography-Mass Spectrum (GC-MS) analysis
Fifty grams of the sample were soaked in absolute ethanol for 48 hours in various portions and repeatedly extracted with ethanol. The combined and concentrated ethanol extract were re-extracted using chloroform to obtain chloroform soluble portion and stored in sample bottle for GC-MS analysis.
GC-MS analysis was carried out using GC-MS QP 2010 Plus Shimazu Japan equipment. An aliquot of 1 μl of the chloroform soluble portion of the sample was injected into the column with injector temperature at 230°C and carrier gas pressure of 100 kpa. The column has a length of 30 m with a diameter of 0.25 mm and a flow rate of 50 ml/min. The eluents were automatically passed into the Mass Spectrometer with a detector voltage set at 1.5 kV and sampling rate of 0.2 seconds. The Mass Spectrometer was also equipped with a computer fed Mass Spectra data bank. The Mass spectrum yielded a spectrum of compounds which was compared with the spectrum of National Institute of Standard and Technology (NIST) database with over 62,000 spectral patterns [10,11]. The identity of the spectra above 95% was used to ascertain the name, molecular weight and structure of the components in the leaves of C. dolichopentalum.
Column chromatographic separation of plant sample
Eight hundred grams of the pulverized samples were soaked in 95% ethanol for 48 hours and filtered. The filtrates were concentrated using rotary evaporator regulated at 40°C to obtain the ethanol extract. The crude ethanol extracts were partitioned between chloroform and water to afford chloroform soluble fractions. Ten grams of the chloroform soluble fractions were subjected to column chromatography over silica gel. The column was eluted as described by Abdelgadir et al. [12] with minor modifications using 100 ml petroleum ether, followed by petroleum ether/chloroform mixture and labelled as follows; (a) 100 ml petroleum ether ACD, (b) 90/10 BCD, (c) 80/20 CCD (d) 70/30 DCD, (e) 60/40 ECD, (f) 50/50 FCD, (g) 40/60 GCD, (h) 30/70 HCD (i) 20/80 ICD (j) 10/90 JCD. Further elution using chloroform and methanol mixture afforded the fraction 90/10 KCD, 80/20 LCD 70/30 MCD, 60/40 NCD, 50/50 OCD, 40/60 PCD, 30/70 QCD, 20/80 RCD 10/90 SCD.
Thin layer chromatography of column eluents
The eluents from column chromatography were subjected to thin layer chromatography following the method of Watanable et al. [13] with minor modifications using silica gel 60 G and iodine vapour for development. This was to select the pure eluents which appeared as a spot from the impure eluents that appeared as more than one spots. Fraction FCD was obtained using petroleum ether/chloroform mixture (3:1) with a Ratio of font (Rf) value of 0.82. Fraction ICD was obtained with Rf value of 0.83 which appeared as a spot. Fraction DCD had Rf value of 0.84, GCD with Rf value of 0.85, JCD with RF value of 0.86, KCD with Rf value of 0.89 and MCD with Rf value of 0.64.
Fourier Transform Infra-Red Spectroscopy (FTIR) analysis
Fourier transform infra-red spectroscopic analysis of the sample was carried out according to the method of Hashimotto et al. and Geethu et al. [14,15]. One gram of the dried plant sample was mixed with 0.5 g of potassium bromate (KBr) and 1.0 ml of nujol oil (a solvent for preparation of sample by Buck 530 IR-spectrophotometer) was added with the aid of a syringe to form a paste. The paste was introduced into the instrument sample mould and allowed to scan at a wavelength of 600-4000 nm to obtain the spectra wavelength. The eluents from the column chromatography were subjected to FTIR analysis; here 1.0 ml of the nujol oil was added to 1.0 ml of the chloroform fraction.
Results and Discussion
Identification of the chemical constituents of plants is important for the discovery of new therapeutic agents [16], and GC-MS is an important tool for this process. Gas chromatography mass spectrometer identifies the bioactive constituents of long chain hydrocarbons, alcohols, acids, ester, alkaloids, steroids, amino and nitro compounds [17]. The chemical characterization of active fraction of tested C. dolichopentalum leaves showed a mixture of fatty acids, nitrocyclohexane, furans, alkanes, organic esters, modified alkanones, alcohols, and eicosanoic acids.
A total of 24 peaks (Figure 1) were observed when the chloroform soluble portion of ethanol extract of C. dolichopentalum leaves was subjected to GC- MS (Table 1). Some of the bioactive compounds include; hexadecanoic acid, Cyclohexamine, Caprolactam, Phenol-2,6- bis(1,1-dimethylethyl)-4, methyl carbamate, 3,7,11,15-Tetramethyl-2- hexadecen-1-ol (Phytol), Heptadecanoic acid.
| S No |
Molecular Weight |
Molecular Weight |
Molecular Formula |
Molecular Structure |
| 1 |
Nitrocyclohexane |
129 |
C6H11NO2 |
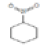 |
| 2 |
Amino caproic lactam |
113 |
C6H11NO |
 |
| 3 |
Phenol-2,6-bis (1,1-dimethyl)-4 -methyl, methyl carbamate |
277 |
C17H27NO2 |
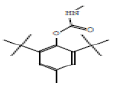 |
| 4 |
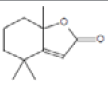
2(4H)-Benzofuranone, 5,6,7,7a-tetrahydro-4,4,7a-trimethyl- |
180 |
C11H16O2 |
 |
| 5 |
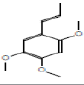
1,2,4-trimethoxy-5-prop-1-enylbenzene |
208 |
C12H16O3 |
 |
| 6 |
4-t-Butyl-2-(1-methyl-2-nitroethyl) cyclohexanone cyclohexanone |
241 |
C13H23NO3 |
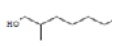 |
| 7 |
2,7- dimethyl-1-octanol |
158 |
C10H22O |
 |
| 8 |
1-Tridecyne |
180 |
C10H22O |
 |
| 9 |
6,10-dimethyl-2-undecanone |
198 |
C13H26O |
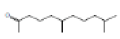 |
| 10 |
Hexadecane |
226 |
C16H34 |
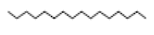 |
| 11 |
Methyl 14-methylpentadecanoate |
270 |
C17H34O2 |
 |
| 12 |
Heptedecanoic acid |
270 |
C17H34O2 |
 |
| 13 |
Ethyl octadecanoate |
312 |
C20H40O2 |
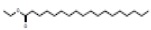 |
| 14 |
Methyl trans-9-octadecanoate |
296 |
C19H36O2 |
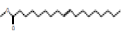 |
| 15 |
3,7,11,15-tetramethyl-2-hexadecen-1-ol |
296 |
C20H40O |
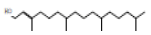 |
| 16 |
11-Hexadecanoic acid |
254 |
C16H30O2 |
 |
| 17 |
Octadecanoic acid |
284 |
C18H36O2 |
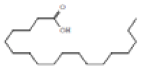 |
| 18 |
1-Flourodecane |
160 |
C10H12F |
 |
| 19 |
Eicosanoic acid |
312 |
C20H40O2 |
 |
| 20 |
Eicosane |
282 |
C20H42 |
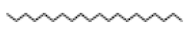 |
| 21 |
9-tetradecenal |
210 |
C14H26O |
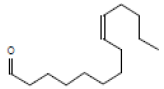 |
| 22 |
2-Buthyl-1-octanol |
186 |
C12H26O |
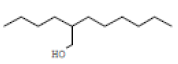 |
| 23 |
Cyclododecane epoxide |
182 |
C12H22O |
 |
| 24 |
Tetratetracotane |
618 |
C44H90 |
 |
Table 1: Molecular weights, formula and structures of compounds identified from the GC-MS of ethanol extract of C. dolichopentalum.
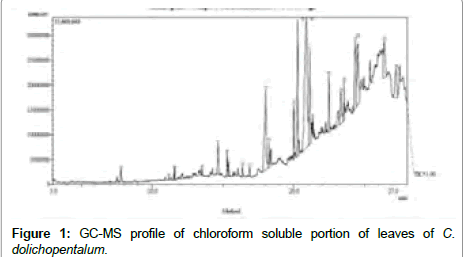
Figure 1: GC-MS profile of chloroform soluble portion of leaves of C. dolichopentalum.
Hexadecanoic acid, a fatty acid with potential antimicrobial and antidiarrhoeal activities [18], is reported to cause growth inhibition and apoptosis induction in human gastric cancer cells [19]. Cyclohexamine belong to an aliphatic amine class. It is used as an intermediate in the synthesis of other organic compounds such as sulphenamide; a base reagent used as accelerators for vulcanization. Cyclohexamine is also used as a building block for pharmaceuticals, e.g., mucolytics, analgesics, and bronchodilators [20].
Phenol-2,6-bis(1,1-dimethylethyl)-4, methyl carbamate could be used to synthesize Phenol-4-[2-(aminomethyl)-4-thiazolyl]-2,6- bis(1,1-dimethylethyl) monohydrochloride which is used for the treatment of Huntington’s disease [21].
The identified hexadecanoic acid ethyl ester has antioxidant activities which are important in biological processes [22,23]. While, 3,7,11,15-Tetramethyl-2- hexadecen-1-ol (Phytol) has antimicrobial and anti-inflammatory activities [23]. Octadecanoic acid (stearic acid) is less likely to be incorporated into cholesterol esters, and thus was found to be associated with lowered LDL cholesterol when compared to other saturated fatty acids in epidemiologic and clinical studies [24]. It is also used to produce dietary supplements [25].
Heptadecanoic acid or margaric acid, is a saturated fatty acid which occurs as a trace component of fat and milk fat of ruminants [26,27].
Ethnobotanical database compounds such as furan, phenols, flavonoids and fatty acid esters possess antioxidant, antibacterial, antimicrobial, anti-inflammatory, antiproliferative, anticancer, antitumor, antidiabetic, antiarthiritic, antimalarial and automatic nerve activities [28,29]. Thus, the presence of these compounds in C. dolichopentalum implies a possibility of exhibiting afore mentioned activities as found in plants such as Litsea glutinosa [30], Suaeda maritime [31], Alpinia hainanesis and Alpinia katsumadai [32], and Macrotyloma uniflorum [33].
The spectra wavelength observed in C. dolichopentalum served as a characteristic medium to elucidate the inherent functional group and organic compounds in the plant [15,34]. The Infra-Red of the pulverized leaves (Table 2) showed different peaks, indicating transitions between vibration levels of different molecules. The peak value at 833 cm-1, was assigned C-Cl stretch of chlorine compound and 1027 cm-1 was assigned CO stretch of ether compounds (Figure 2). The medium bands at 1257 cm-1, 1632 cm-1, 3405 cm-1 were thus assigned NH stretch of amine compounds. The weak band at 1876 cm-1, and 2060 cm-1 were assigned CO stretch of unsaturated ester and carboxylic compounds. Also, 1454 cm-1, 2753 cm-1, 2856 cm-1, 2570 cm-1 and 2664 cm-1 peaks were assigned CH and SH symmetric stretch of methylene and thiol compounds. The broad band at 3065 cm-1, 3160 cm-1, and 3548 cm-1 were assigned OH stretch of primary (1°) and tertiary (3°) alcohol compounds [15,35].
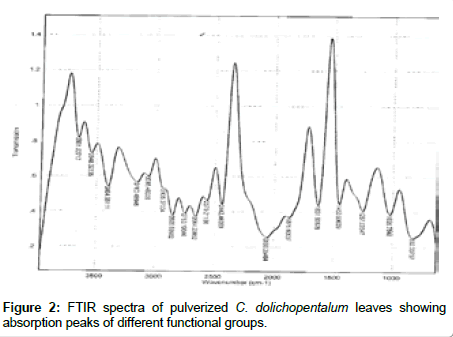
Figure 2: FTIR spectra of pulverized C. dolichopentalum leaves showing absorption peaks of different functional groups.
| S No |
Wavelength (cm-1) |
Functional Group |
Compounds |
| 1 |
833 |
C-Cl |
Chloro compound C-Cl stretch |
| 2 |
1027 |
R-O-P |
Ether CO stretch |
| 3 |
1257 |
RNH3 |
10 amine NH stretch |
| 4 |
1454 |
CH3 |
Methyl CH stretch |
| 5 |
1632 |
RNH2 |
20 amine NH stretch |
| 6 |
1876 |
C-O-C |
Cyclic ester CO stretch |
| 7 |
2060 |
RCOOH |
Carboxylic acid COO stretch |
| 8 |
2442 |
R-C=N |
Nitriles CN anti-symmetric stretch |
| 9 |
2570 |
CH2SH |
Thiol SH stretch |
| 10 |
2664 |
CH2SH |
Thiol SH stretch |
| 11 |
2753 |
CH2 |
Methylene CH stretch |
| 12 |
2856 |
CH2 |
Methylene CH stretch |
| 13 |
2935 |
R-S-C=N |
Thiocyanate SCN anti-symmetric stretch |
| 14 |
3065 |
RCHOH |
10 alcohol OH stretch |
| 15 |
3160 |
RCHOH |
10 alcohol OH stretch |
| 16 |
3405 |
RNH2 |
20 amine NH stretch |
| 17 |
3548 |
R2CHOH |
30 alcohol OH stretch |
| 18 |
3662 |
R3N |
30 amine NH stretch |
Table 2: FTIR spectra characteristics of crude ethanol extract of C. dolichopentalum.
The Infra-Red of the seven eluates are shown in Figures 3-9. The interpretation of the IR spectra of the eluates using the GC-MS spectra indicated Dcd eluate (Figure 3) as 4-t-Butyl-2(1-methyl-2-nitroethyl) cyclohexanone, with the Rf value of 0.84, molecular weight- 234 and an empirical formular C13H23NO3. N-H stretch occurs in the range 3500- 3300 cm-1. Eluate Fcd (Figure 4) as flourodecane an alkane which IR spectrum are usually simple with few peaks. C-H stretch occur around 3000 cm-1 and alkanes (except strained ring compounds) absorption always occurs to the right of 3000 cm-1. Gcd eluate (Figure 5) was identified as phenol-2, 6- bis (1,1-dimethyl ethyl)-4, methyl carbamate with the empirical formular C17H27NO2. Icd (Figure 6) and Kcd (Figure 7) eluates were identified as pentadecane (C15H32) and hexadecane (C16H34) respectively. Eluates Jcd (Figure 8) and Mcd (Figure 9) as 2, 7-dimethyl-1-1octanol (C10H22O) and 2-Butyl-1-octanol (C12H26O) respectively [10,11,36-38].
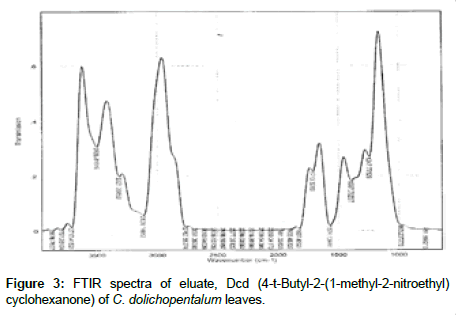
Figure 3: FTIR spectra of eluate, Dcd (4-t-Butyl-2-(1-methyl-2-nitroethyl) cyclohexanone) of C. dolichopentalum leaves.
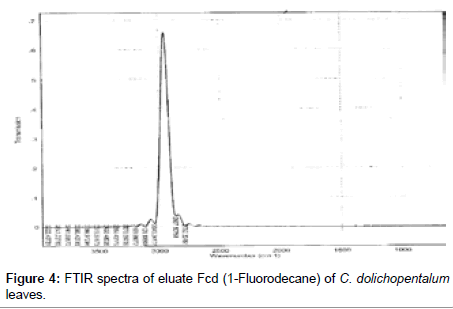
Figure 4: FTIR spectra of eluate Fcd (1-Fluorodecane) of C. dolichopentalum leaves.
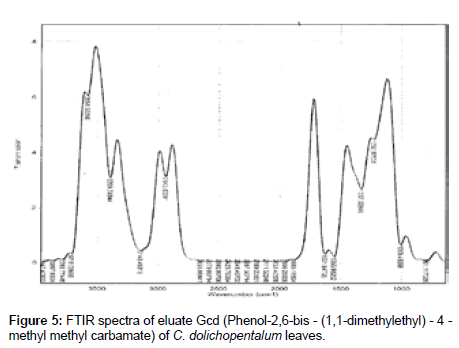
Figure 5: FTIR spectra of eluate Gcd (Phenol-2,6-bis - (1,1-dimethylethyl) - 4 - methyl methyl carbamate) of C. dolichopentalum leaves.
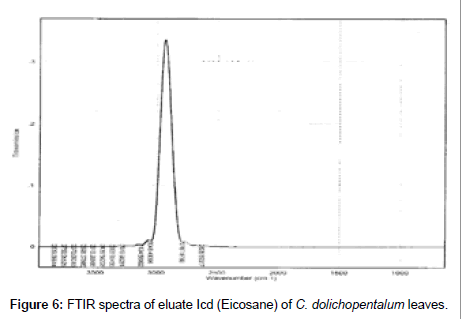
Figure 6: FTIR spectra of eluate Icd (Eicosane) of C. dolichopentalum leaves.
Figure 7: FTIR spectra of eluate Jcd (2,7-Dimethyl-1-octanol), of C. dolichopentalum.
Figure 8: FTIR spectra of eluate Kcd (Hexadecane) of C. dolichopentalum leaves.
Figure 9: FTIR spectra of eluate Mcd (2-Butyl-1-octanol) of C. dolichopentalum leaves.
The present study showed that leaves of C. dolichopentalum contain a variety of biologically active phytoorganic compounds by using GC-MS and IR spectroscopy as pharmacognostic tool for the identification of plant constituents. The beneficial roles of these bioactive phytoorganic constituents can be harnessed and used in the pharmaceutical and food industries for the production of drugs and raw materials for industrial purposes.
Acknowledgements
The authors appreciate the technical support and contributions of Prof. Ukoha AI of the Department of Biochemistry and Dr. IC Iwu of the Department of Chemistry, Federal University Technology Owerri, Nigeria. The authors appreciate Mr. A Ozioko of the Bioresource Development and Conservation Program (BDCP), Research Centre, Nsukka, Enugu State and Dr. FN Mbagwu of the Department of Plant Science and Biotechnology, Imo State University, Owerri, Nigeria for their assistance in identifying and providing voucher number for the plant.
19512
References
- Mahesh B, Satish S (2008) Antimicrobial activity of some important medicinal plant against plant and human pathogens. World J Agric Sci 4: 839-843.
- Igwe CU, Nwaogu LA, Ujowundu CO (2007) Assessment of the hepatic effects, phytochemical and proximate compositions of Phyllanthus amarus. Afr J Biotech 6: 728-731.
- Ojiako OA, Nwanjo HU (2009) Biochemical studies of the effects of the aqueous extract of Nigerian garlic on lipid profile and atherogenic risk predictor indices. Aust J Basic Applied Sci 3: 2861-2865.
- Nwaoguikpe RN, Braide W, Ujowundu CO (2012) Phytochemicals, minerals, vitamins and antioxidant compositions of three Nigerian medicinal plants. Instasci J Pharma Sci 2: 50-57.
- Janakiraman N, Johnson M, Sahaya SS (2012) GC-MS analysis of bioactive constituents of Peristrophe bicalyculata (Retz.) Nees. (Acanthaceae). Asian Pac J Trop Biomed S46-S49.
- Balamurugan K, Nishanthini A, Mohan VR (2012) GC-MS analysis of Polycarpaea corymbosa(L.) Lam whole plant. Asian Pac J Trop Biomed S1289-S1292.
- Ncube NS, Afolayan AJ, Okoh AI (2008) Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol 7: 1797-1806.
- Kalu FN, Ogugua VN, Ujowundu CO, Chinekeokwu CRK (2011) Phytochemical composition and acute toxicity study on aqueous extract of Combretum dolichopentalum leaf in Swiss Albino mice.Res J Chem Sci 1: 72-75.
- Ujowundu FN, Ukoha AI, Ojiako AO, Nwaoguikpe RN (2015) Isolation of bioactive phytochemicals in leaves of Combretum dolichopentalum and their hydrogen peroxide scavenging potentials. Pharma Analytica Acta 6: 11.
- McLafferly M (1989) Registry of mass spectral data. 5th edn. Wiley, New York, USA.
- Stein S (1990) National Institute of Standards and Technology (NIST) Mass Spectral Database and Software.Version 3.02, USA.
- Abdelgadir AA, Ahmed EM, Eltohami MS (2011) Isolation, characterization and quantity determination of aristolochic acids, toxic compounds in Aristolochia bracteolata L. Environ Health Insights 5: 1.
- Watanable K, Miyakado M, Iwai T, Izumi K, Yanagi K (1988) Isolation of aristolochic acid and aristolic acid from Cocculus triolobus dc as potent seed germination inhibitor. Agric Biol Chem 52: 1079-1082.
- Hashimoto A, Kameoka T (2008) Applications of infrared spectroscopy to biochemical, food, and agricultural processes. Appl Spectroscopy Rev 43: 416-451.
- Geethu MG, Suchithra PS, Kavitha CH, Aswathy JM, Dinesh Babu D, et al. (2014) Fourier-transform infrared spectroscopy analysis of different solvent extracts of water Hyacinth (Eichhornia Crassipes Mart Solms.) an allelopathic approach. World J Pharm Sci 3: 1256-1266.
- Milne A (1993) Inhalational and local anesthetics reduce tactile and thermal responses in Mimosa pudicalinn. Masui1190-1193.
- Singariya P, Kumar P, Mourya KK (2012) Identification of New Bioactive Compounds by GC-MS and Estimation of Physiological and Biological Activity of Kala Dhaman (Cenchrus setigerus). Inter J Pharma Biol Arch 3: 610-616.
- Mamza UT, Sodipo OA, Khan IZ (2012) Gas chromatography-mass spectrometry (GC-MS) analysis of bioactive components of phyllanthus amarusleaves. Inter Res J Plant Sci 3: 208-215.
- Daniet AO, Folahan OA, Ayele G, Adrian A, Ernest BI, et al. (2011) Biological activity and mass spectrometric analysis of Vernonia amygdalinaFractions. J Biosci Technol, pp: 287-304.
- Karsten E, Erhard H, Roland R, Hartmut H (2005) Amines, Aliphatic in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim.
- Committee for Orphan Medicinal Products (2015) Public Summary of Opinion of Orphgan designation. Phenol, 4- (2-(amino methyl)-4- thiazoyl)-2, 6-bis (1,1- dimethylethyl) monohydrochloride for the treatment of Huntington’s disease.
- Cluette-Brown JE, Khan ZA, Hasaba H, Lopez de Heredia L, Laposata M (2005) Fatty acid methyl esters are detectable in the plasma and their presence correlates with liver dysfunction. Clin Chimica Acta 359: 141-149.
- Sudha T, Chidambarampillai S, Mohan VR (2013) GC-MS analysis of bioactive components of aerial parts of kirganelia reticulatapoir (euphorbiaceae). J Curr Chem Pharm Sci 3: 113-122.
- Hunter JE, Zhang J, Kris-Etherton PM (2009) Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: A systematic review. Amer J Clin Nutr 91: 46-63.
- Ram Charan Company (2013) Applications of Industry Based Stearic Acid. Product Development Information, India 3: 1-2.
- Beare-Rogers J, Dieffenbacher A, Holm JV (2001) Lexicon of lipid nutrition (IUPAC Technical report). Pure Appl Chem 73: 685-744.
- Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, et al. (2010) Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51: 3299-305.
- Gopalakrishnan K, Udayakumar R (2014) GC-MS Analysis of Phytocompounds of leaf and stem of Marsilea quadrifolia (L.). International J Biochem Res Rev 4: 517-526.
- Tamilselvan V, Rajeswari M, Velayutham P (2014) GC-MS analysis and "in vitro" anticancer activity of methanolic root extract of asystasia gangetica (L). World J Pharmacy Pharma Sci 12: 957-967.
- Chowdhury JU, Bhuiyan MNI, Nandi NC (2008) Aromatic plants of Bangladesh: Essential oils of leaves and fruits of Litsea glutinosa(Lour.). Bangladesh J Bot 37: 81-83.
- Leach RP, Wheeler KP, Flowers TJ, Yeo AR (1990) Molecular markers for ion compartmentation in cells of higher plants: II. Lipid composition of the tonoplast of the halophyte Suaeda maritime(L.). Dum J Exp Bot 41: 1089-1994.
- Nan P, Hu Y, Zhao J, Feng Y, Zhong Y (2004) Chemical composition of the essential oils of two Alpiniaspecies from Hainan island. China. Z Naturforsch 59: 153-160.
- Yesu RJ, Paul M, John P, Joy V (2012) Chemical compounds investigation of Cassia auriculata seeds: A potential folklore medicinal plant. Asian J Plant Sci Res 2: 187-192.
- Dias DA, Jones OAH, Beale DJ, Boughton BA, Benheim D, et al. (2016) Current and future perspectives on the structural identification of small molecules in biological systems. Metabolites 6: 46.
- Ashokkumar R, Ramaswamy M (2014) Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int J Curr Microbiol App Sci 3: 395-406.
- Saranya D, Sekar J (2016) GC-MS and FT-IR Analyses of Ethylacetate Leaf extract of Abutilon indicum(L.) Sweet. Int J Adv Res Biol Sci 3: 193-197.
- Duke JA, Ayensu ES (1985) Medicinal plants of China. Reference Publications.
- Pavia DL, Lampman GM, Kriz GS (1982) Introduction to Organic Laboratory Technique. Nicotine. 2nd edn., pp: 48-49.