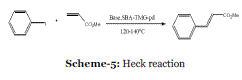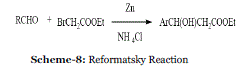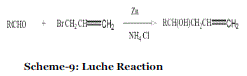Key words
|
| |
| Solvent less reaction, Greener reaction, Michael addition, Heck reaction, Reformatsky reaction, dihydropyrimidones |
| |
INTRODUCTION
|
| |
| The earlier belief that no reaction is possible without the use of a solvent is no more valid. It has been found that a large number of reactions occur in solid state without the solvent. Infact in a number of cases, such reactions occur more efficiently and with more selectivity compared to reactions carried out in solvents. Such reactions are simple to handle, reduce pollution, comparatively cheaper to operate and are especially important in industry. It is believed that solvent free organic synthesis and transformations are industrially useful and largely green. Green chemistry, also called sustainable chemistry, is a chemical philosophy encouraging the design of products and processes that reduce or eliminate the use and generation of hazardous substances. It covers such concepts as: |
| |
| · The design of process to maximize the amount of raw material that ends up in the product |
| |
| · The use of safe, environment benign substances, including solvents, whenever possible |
| |
| · The design of energy efficient processes |
| |
| · The best form of waste disposal: do not create it in the first place. |
| |
| Prof. Paul Anastas of the United States Environmental Protection Agency and Prof. John C. Warner gave 12 principles of green chemistry: |
| |
| 1. It is better to prevent waste than to treat or clean up waste after it is formed. |
| |
| 2. Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product. |
| |
| 3. Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment. Chemical products should be designed to prevent efficacy of function while reducing toxicity. |
| |
| 4. The use of auxiliary substances (solvents, separation agents, etc,) should be made unnecessary whenever possible and innocuous when used. |
| |
| 5. Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure. |
| |
| 6. A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. |
| |
| 7. Unnecessary derivatization (blocking group, protection/deprotection, and temporary modification of physical/chemical processes) should be avoided whenever possible. |
| |
| 8. Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. |
| |
| 9. Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degradation products. |
| |
| 10.Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. |
| |
| 11. Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fires. |
| |
| 12. Inherently Safer Chemistry for Accident Prevention: Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires. |
| |
| Solid state reaction follows the fifth principle of green chemistry which avoid using solvents and chemical reactions mostly occurring at room temperature which lead to energy efficiency. |
| |
|
Solid-state Reactions
|
| |
| A dry media reaction or solid-state reaction or solventless reaction is a chemical reaction system in the absence of a solvent.1 The drive for the development of dry media reactions in chemistry is: |
| |
| · Economics (save money on solvent) |
| |
| · Not required to remove a solvent after reaction completion ultimetly purification step not required |
| |
| · Reaction rate is high due more avaibility of reactants |
| |
| · Environmentaly friendly because solvent is not required |
| |
| · Some of the drawbacks are: |
| |
| · Homogenous reactants should mix to a system |
| |
| · Viscosity high in reaction sysyem |
| |
| · Unsuitable for solvent assisted chemical reactions |
| |
| Nevertheless, it is remarkable that chemists still carry out their reactions in solution, even when a special reason for the use of solvent cannot be found.2 It has been found that many reactions proceed efficiently in the solid state. The occurrence of efficient solid-state reactions shows that the molecules reacting are able to move freely in the solid state. In fact, host-guest inclusion complexation can occur by simply mixing and grinding both crystals in the solid state. These solid-state reactions can be easily monitored by measurement of IR and UV spectra in the solid state. Though it is a common practice to run the organic reactions in solvent media, the chemists concern to minimize the environmental pollution caused by solvents and also their academic interest in solid-solid reactions have led them in recent times to develop methodologies for solvent-free reactions with considerable success. |
| |
|
The Function of a Solvent
|
| |
| A general assumption with regard to organic reactions is that they are performed in a solvent medium. The rationale behind this concept is simple. That is, the reactants can interact effectively if they are in a homogeneous solution, which facilitates the stirring, shaking or other ways of agitation, whereby the reactant molecules come together rapidly and continuously. Moreover, uniform heating or cooling of the mixture, if needed, can be carried out in a solution relatively easily. However, the role of a solvent in the context of an organic reaction is much more complex than merely providing a homogeneous setting for a large number of collisions of the reactants to take place. A solvent has the power to enhance or reduce the speed of a reaction, at times enormously. Changing of solvent of a reaction can influence the rate of that reaction and it can be powerful enough to change the reaction course itself. This may manifest in altered yields and ratios of the products. Thus a solvent could be deeply and inseparably associated with the process of an organic reaction through the solvation of the reactants, products, transition-state or other intervening species. Such intimate interactions between the solvent and the reaction partners are due to many factors that include electrostatic, steric and conformational effects, among others. In spite of such a strong involvement, the solvent does not normally become part of the product, except in the case of solvolysis reactions and is recovered unchanged after the reaction is over. Even then, one may not envisage or plan to perform a reaction in the absence of a solvent. |
| |
|
Liquid as a Solvent
|
| |
| In principle, any liquid can be used as a solvent. However, the number of commonly used solvents is severely restricted. They include a few hydrocarbons, chlorinated hydrocarbons, a few ethers, esters, alcohols, amide derivatives, sulphoxides, etc. liquid ammonia, carbon disulfide and of course water, are also frequently used as medium to carry out synthesis. The suitability of a solvent for a reaction depends on many factors. A solvent should be selected for a new reaction based on its physical and chemical properties. At times the liquid reactant itself would serve as solvent. In any case, a solvent is usually considered to be an inevitable component of a reaction. A reaction under solvent free condition or in solid state was generally thought to be not quite feasible, or at least not quite efficient, though several solid state organic reactions have been known for a long time. However, the chemists concern for developing environment-friendly synthetic procedures has made them turns their attention to minimize or circumvent the use of solvents that are a major cause of pollution. This has led, in recent times, to vigorous research activity and reinvestigation of known reactions to achieve organic synthesis under solvent-free condition.3,4 |
| |
|
Advantages of Solvent-free Reactions
|
| |
| · A solvent-free or solid state reaction may be carried out using the reactants alone or incorporating them in clays, zeolites, silica, alumina or other matrices. Thermal process or irradiation with UV, microwave or ultrasound can be employed to bring about the reaction. |
| |
| · Solvent-free reactions obviously reduce pollution and bring down handling costs due to simplification of experimental procedure, work up technique and saving in labour. These would be especially important during industrial production. |
| |
| · Often, the products of solid state reactions turn out to be different from those obtained in solution phase reactions. This is because of specific spatial orientation or packing of the reacting molecules in the crystalline state. The orientational requirements of the substrate molecules in the crystalline state have provided excellent opportunities to achieve high degree of stereoselectivity in the products. This has made it possible to synthesize chiral molecules from prochiral ones either by complexation with chiral hosts or formation of intermediates with chiral partners. |
| |
| · If two or more substrates are involved in the reaction, they are thoroughly ground together in a glass mortar or cocrystallized and allowed to stay at room temperature or transferred to a suitable apparatus and heated carefully in an oil bath or exposed to appropriate radiation until the reaction is complete. More sophisticated reaction procedures are also adopted, if necessary. The progress of the reaction can be monitored by TLC. In some cases, a small quantity of water or a catalyst may be added. If it is a single-compound reaction, it is subjected to heat or radiation directly. Care is to be taken to collect the volatile products, if they are produced. |
| |
| Greener Reactions under solventless conditions Due to the growing concern for the influence of the organic solvent on the environment as well as on human body, organic reactions without use of conventional organic solvents have attracted the attention of synthetic organic chemists. Although a number of modern solvents, such as fluorous media, ionic liquids and water have been extensively studied recently, not using a solvent at all is definitely the best option. Development of solvent-free organic reactions is thus gaining prominence. |
| |
| Event-1: Catalyst and solvent-free conditions as an environmentally benign approach to 4- aryl-3-cyano-hexahydro-4H-1,2-benzoxazine- 2-oxides: 1,2-Oxazine-2-oxides are generally accessible via a catalyzed formal [4+2] cycloaddition reactions of nitroalkenes with electron-rich alkenes.5,6 These heterocycles are valuable intermediates to prepare in a regio and stereoselectively manner a number of important building blocks or target heterocyclic compounds, such as pyrrolidines, β-lactam-N-oxides, pyrrolizidine and indolizidine alkaloids, enamines, ketoalcohols, nitroketones, etc. |
| |
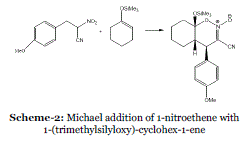
| In 2008, Bellachioma et.al reported that (E)-2-aryl- 1-cyano-1-nitroethenes are excellent Michael acceptors in water in the reactions with enantiopure alkyl vinyl ethers, allowing the preparation of various cyclic nitronates by a completely endo stereoselective [4+2] cycloaddition [10] (Scheme 1).7-9 |
| |
| Under solvent-free conditions, (E)-2-aryl-1-cyano-1- nitroethenes rapidly react with 1-(trimethylsilyloxy)- cyclohex-1-ene with a complete regio- and diasteroselectivity and leading to the exclusive formation of the cis-fused hexahydro-4H-benzoxazine-2-oxides, which were isolated without the need for a work-up procedure in excellent yields. |
| |
| Event-2: Transesterifications catalysed by solid, reusable apatite–zinc chloride: Transesterification is an important organic reaction that can be used to synthesize various intermediates in the synthesis of complex natural products, pheromones and paint additives.10,11 Its value has been considerably enhanced by its use as a key step in the manufacture of biodiesel, one of the more environmentally friendly and sustainable fuels we have available today. |
| |
| Transesterification can be catalysed by strong acids and by soluble bases such as caustic soda, neither of which is ideal from a green chemistry perspective.12-14 Rather, there should be safe to handle and store, environmentally benign and reusable, typically solid catalysts. This has led to reports of a number of active synthetic basic solids as transesterification catalysts, including mixed metal hydrotalcites and zeolites. Drawbacks with such catalysts include the use of hazardous reagents in the preparation of the catalyst (e.g. KOtBu) and pore blockage with larger substrates.15,16 Hydroxyapatite [HAP; Ca10(PO4)6(OH)2] is a highly abundant, natural material, being the major component of teeth and bones. It is also considered to have durable acid–base properties and high adsorption capacity, making it a promising support material. This has been demonstrated in the use of HAP-supported Lewis acids in Friedel–Crafts alkylations and more recently in Michael addition of indoles to electron-deficient olefins.17,18 In 2006, Solhy et. al report for the first time the use of low cost and safe to handle fluoroapatite and hydroxyapatite supported zinc chloride reagents as solid and reusable catalysts for a wide range of transesterification reactions19. |
| |
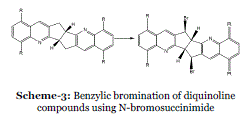
| Event-3: Solid-state regio and stereo-selective benzylic bromination of diquinoline compounds using Nbromosuccinimide: The Wohl–Ziegler reaction, namely allylic or benzylic free radical bromination using Nbromosuccinimide (NBS) in a refluxing aprotic solvent, (Scheme 3) is a well-established synthetic organic procedure.20 Benzene, chloroform and petrol have been employed as solvents, but the traditional choice has been carbon tetrachloride which combines optimum properties of solubility, reaction temperature and ease of product isolation. The succinimide by-product can be removed simply by filtration of the cooled reaction mixture and then evaporation of solvent from the filtrate affords the brominated product.21 |
| |
| In 2005, Rahman et al. synthesised a series of new brominated diquinoline lattice inclusion hosts, some of which have potential in separation chemistry due to their selective properties. In each case, the final step involved a regio and stereoselective benzylic NBS bromination in refluxing CCl4. However, identical products can be obtained by means of solid-state reaction.22 |
| |
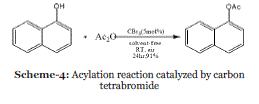
| Event-4: Metal and solvent-free conditions for the acylation reaction catalyzed by carbon tetrabromide: Organocatalysis has attracted much attention as result of both the novelty of the concept and more importantly the fact that the efficiency and selectivity of many organocatalytic reactions meet the standards of established organic reactions. Catalysts of the same class may promote similar reactions or less closely related reactions e.g., Carbon tetrabromide (CBr 4 ) is another example of this catalyst class is able to mediate an astonishing variety of transformations. Although carbon tetrabromide is considered a poisonous, irritating solid (skin contact can cause severe irritation; avoid inhalation of fumes; toxicity: irritating to skin, eyes and respiratory tract, irritating to mucous membranes, narcotic in high concentrations; possible liver and kidney damage;). It has been utilized as a mild Lewis acid imparting high regio and chemo-selectivity in various organic transformations.23 In 2007, Zhang et al. reported that an efficient and useful catalyst carbon tetrabromide (CBr4) was discovered to be highly effective for the acylation of phenols, alcohols and thiols under metal and solvent-free conditions (Scheme 4).24 |
| |
| Event-5: Solvent-free Heck reaction catalyzed by a recyclable Pd catalyst supported on SBA- 15 via an ionic liquid: The palladium-catalyzed coupling of olefins with aryl or vinyl halides, known as the Heck reaction, is one of the most powerful methods to form a new carbon–carbon (Csp 2 –Csp 2 ) bond in modern synthetic chemistry. The Heck reaction provides a direct route to assemble important arylated and vinylated olefins and its wide functional group tolerance on both reactants allows the convenient application at a late stage of total synthesis without protecting groups. Since its discovery by Heck et al. in the late 1960s, the reaction has been extensively studied and applied to a wide variety of fields, such as natural product synthesis material science and bioorganic chemistry.25-29 A major transformation in the Heck reaction is the synthesis of cinnamic acid and its derivatives, which are important intermediates in the synthesis of medical products and commonly used as flavor substances and UV absorbents. The reaction is usually catalyzed by palladium complexes in a homogeneous mode of operation and shares common drawbacks i.e., catalyst recovery and recycling is difficult. In addition, expensive, generally unstable and toxic ligands, such as phosphine, are required to activate and stabilize Pd against agglomeration and formation of Pd black.30 Therefore, cheaper and environmentally benign heterogeneous catalytic systems are attractive, and notable progress has been made in this area. Palladium supported on a diverse array of organic and inorganic materials, such as polymers, carbon, metal oxides, clay, ordered or amorphous silicates and zeolites have been developed and used to catalyze the Heck reaction. |
| |
| The Heck reaction can be performed in organic solvents, ionic liquids and CO2 Scheme 5: Palladiumcatalyzed coupling of olefins with aryl or vinyl halides. However, less attention has been paid on the Heck reaction in a solvent-free condition, although it is environmentally benign and economically profitable. In 2008, Jinag et al. reported that Heck arylation of olefins with aryl halides was carried out in solvent-free conditions with a Pd catalyst supported on 1,1,3,3-tetramethylguanidinium (TMG) modified molecular sieve SBA-15 (designated as SBATMG- Pd) (Scheme 4).31 SBA-TMG-Pd was found to be much more active and stable than a Pd catalyst supported on pristine SBA-15 (designated as SBAPd). These catalysts were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy (TEM) and the reasons for the excellent performance of catalyst SBA-TMG-Pd were also discussed. |
| |
| Event-6: An environmentally benign solventfree Tishchenko reaction: The conversion of aldehydes to their dimeric esters, better known as the Tishchenko reaction has been known for more than a hundred years. This reaction is heavily used in industry and it is inherently environmentally benign since it utilizes catalytic conditions and is 100% atom economic. Over the years, chemists have looked to develop new reagents that are more efficient than the aluminum based catalysts traditionally used. Metal catalysts such as alkali metals, alkali metal oxides, lanthanides, and many others have been developed towards the improvement of Tishchenko chemistry. In 2009, Waddell et al. reported that the solvent-free ball milling Tishchenko and Waddell reaction.32 Using high speed ball milling and a sodium hydride catalyst, the Tishchenko reaction was performed for aryl aldehydes in high yields in 0.5 hours (Scheme 5). The reaction was not affected by the type of ball bearing used and found to be successful in a liquid nitrogen environment. |
| |
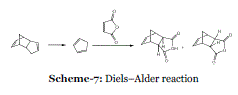
| Event-7: Solvent-free Diels–Alder reactions of in situ generated cyclopentadiene: In 2009, Huertas et al. reported a solvent-free Diels–Alder reaction by heating a mixture of dicyclopentadiene and a dienophile (Scheme 7). Cyclopentadiene, formed in situ, reacted with the dienophile in a thermodynamically controlled reaction.33 Besides being solvent-free; the procedure allows for almost complete utilization of dicyclopentadiene and avoids handling of noxious and hazardous cyclopentadiene. The reaction was found to work well with dienophiles such as maleic anhydride and unsaturated esters. However, unsaturated acids were not suitable dienophiles, as Diels–Alder adducts was obtained in a low yield. |
| |
| Advantages of this procedure are that cyclopentadiene reacts as it is generated and thus there are neither safety problems associated with use of cyclopentadiene nor problems with formation of oligomers. As a result, most of the starting dicyclopentadiene is utilized. Furthermore, as the reaction was performed at a relatively high temperature (162–206°C), thermodynamically preferred reaction products are obtained. Ordinarily, Diels–Alder reactions involving cyclopentadiene proceed under kinetic conditions to give predominantly or exclusively the endo isomer. By adjusting the reaction times, one can obtain either the exo or endo isomer as the major reaction product. By avoiding use of a reaction solvent this is a “greener” procedure compared to traditional Diels– Alder reactions. |
| |
| Event-8: Reformatsky and Luche Reaction in absence of solvent: In 1990, Tanaka et al. reported Reformatsky (Scheme 8) and Luche reactions (Scheme 9) with Zn provide more economical C-C bond formation methods than Grignard reactions with more expensive Mg metal.34 |
| |
|
Scheme-9: Luche Reaction
|
| |
| In addition, it was pointed out that Reformatsky and Luche reactions proceed efficiently in the absence of solvent, although Grignard reactions under similar conditions are not very efficient and give more reduction product than the normal carbonyl addition product. The nonsolvent Reformatsky and Luche reactions can be carried out by a very simple procedure and give products in higher yield than with solvent. In general, the nonsolvent reaction was carried out by mixing aldehyde or ketone, organic bromo compound and Zn-NH4 Cl in an agate mortar and pestle and by keeping the mixture at room temperature for several hours. |
| |
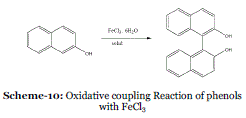
| Event-9: Oxidative coupling Reaction of phenols with FeCl3: Oxidative couplings of phenols are usually carried out by treatment of phenols in solution with more than equimolar amount of metal salts such as FeCl3 or manganese tris(acetylacetonate), although the latter one is too expensive to use in a large quantity. The coupling reactions of phenols with FeCl3, some times quinines as byproducts. |
| |
| In 1989, Toda et al. have reported that some oxidative coupling reactions of phenols with FeCl3 are faster and more efficient in the solid state than in solution (Scheme 10). Some coupling reactions in the solid state were accelerated by irradiation with ultrasound. Some coupling reactions are achieved by using a catalytic amount of FeCl3.35 |
| |
|
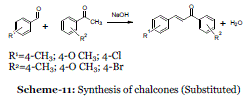
Event-10: Solvent free synthesis of chalcones:
|
| |
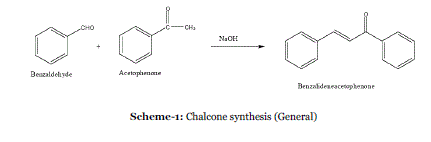
| The synthesis of chalcones illustrates the reaction that proceeds with high atom economy and is relatively easy to perform in teaching labs. Chalcones are important compounds with applications in medicine and physics. |
| |
| In 2004, Palleros et al. found that the reactions proceed rapidly and afford very good yields of product. Most of the chalcones can be obtained in a matter of minutes by mixing the corresponding benzaldehyde and acetophenone in the presence of solid NaOH in a mortar with pestle (Scheme 11); the yields of crude product were in the range 81–94%.36 |
| |
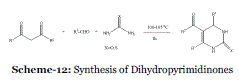
| Event-11: A Practical and Green Approach towards Synthesis of Dihydropyrimidinones without Any Solvent or Catalyst: In 2002, Ranu et al. reported a simple, efficient, green and costeffective procedure for the synthesis of dihydropyrimidinones by a solvent-free and catalystfree Biginelli’s condensation of 1,3-dicarbonyl compound, aldehyde and urea (Scheme 12). This approach of direct reaction in neat without solvent and catalyst showed a new direction in green synthesis.37 |
| |
| Dihydropyrimidinone derivatives are of considerable interest in industry as well as in academia because of their promising biological activities as calcium channel blockers, antihypertensive agents and anticancer drugs. Thus, synthesis of this heterocyclic nucleus is of much importance and quite a number of synthetic procedures based on the modifications of the century-old Biginelli’s reaction involving acidcatalyzed three-component condensation of 1,3- dicarbonyl compound, aldehyde and urea, have been developed. Basically these methods are all similar using different Lewis acid catalysts such as BF 3 , FeCl 3 , InCl 3 and 6h in a solvent such as CH 3 CN, THF. A number of procedures under solvent-free conditions using Yb(OTf) 3 , montmorillonite and ionic liquid as catalysts have also been reported. Obviously, many of these catalysts and solvents are not at all acceptable in the context of green synthesis. Thus, as a part of green synthesis, they have discovered that Biginelli’s reaction proceeds very efficiently by stirring a mixture of neat reactants at 100-105°C for an hour, requiring no solvent and catalyst, and producing dihydropyrimidinones in high yields. |
| |
Conclusion
|
| |
| Green chemistry in ecofriendly safe reaction follows the solvent-free or solid state reaction carried out using the reactants alone or incorporating them in clays, zeolites, silica, alumina or other matrices to achieve high degree of stereoselectivity in the products, to reduce byproducts, to maximize rate of reaction. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Figures at a glance
|
 |
 |
 |
 |
| Scheme 1 |
Scheme 2 |
Scheme 3 |
Scheme 4 |
|
| |
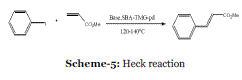 |
 |
 |
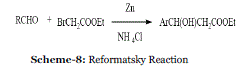 |
| Scheme 5 |
Scheme 6 |
Scheme 7 |
Scheme 8 |
|
| |
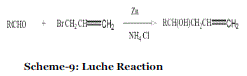 |
 |
 |
 |
| Scheme 9 |
Scheme 10 |
Scheme 11 |
Scheme 12 |
|
| |