Keywords
|
| Glioblastoma multiforme, Survival, Temozolomide, Radiotherapy, Gross total resection |
Introduction
|
| Glioblastoma multiforme (GBM) is the most aggressive glioma subtype (with high proliferative activity) and the most frequent type of primary brain tumors (2% of all adult cancers with median survival of about a year) [1-3]. The incidence in Europe and North America is 2-3 / 100.000 per year [4]. |
| Treatment with combination of surgery, radiation and chemotherapy, still constitutes a challenge in order to extend patient’s life [5-7]. That’s mainly because this kind of tumor is extremely infiltrated, so its gross total resection is difficult [8]. Many experimental therapies have been proposed, but they all, in general, have been failed to succeed a better outcome. So, median survival still remains at low rates, since diagnosis established [6,8]. Angiogenesis and invasive ability of the glial cells into the white matter tracts have been implicated as a great reason for tumor resistance [5]. Survival benefits of the combination of Radiotherapy (RT) and Temozolomide (TMZ) have been demarcated by many studies [9-12]. Despite many efforts and aggressive therapies, including O6-alkylating agents, the detouring of blood-brain barrier and strategies targeting the altered signaling pathways and the glioma-initiating cells, GBMs exhibit a poor prognosis [6]. The most effective management of GBM, includes the safer maximal resection of the tumor [13]. |
| Objective of this study is to find out, if there is any benefit from the combination of Gross Total Resection (GTR) of the tumor and postoperative management with Radiotherapy and Temozolomide (RT&TMZ). |
Material and Methods
|
| It was designed a 5 years retrospective study. Data included patients 15 years old or older, who were admitted to the Department of Neurosurgery, of University Hospital of Larissa in Greece, between January 2007 and December 2011. It was male and female, with GBM diagnosis, established preoperative by Magnetic Resonance Spectroscopy (MRS). All of them underwent surgical resection of the tumor, using Microscope, Neuro-navigation and Evoked Potentials assistance. |
| After the surgical removal of the tumor, there were two different options for the patients: treatment with combination of RT and TMZ, or doing nothing more. |
| From the study were excluded patients younger than 15 years old and these underwent conservative treatment. From patients who underwent surgical resection of the tumor, were excluded those without Neuro-navigation assistance and those with other than positive GBM biopsy. Moreover, were excluded patients underwent more than one surgical intervention of the tumor and those with different treatment strategies than the three was mentioned above. |
| Collected information included sex, age, Ki-67 levels, preoperative Karnofsky performance score (KPS), therapeutic approach and survival time. Ki-67 is a proliferation associated protein, which takes active part in all cell cycle phases, except from G0. So, it can be used as a marker for the determination of a tumor cell population [14,15]. KPS is a valuation score, used for the classification of patients’ functional impairment. This scale, scores from 0 (death) to 100 (no evidence of disease) [16-21]. There is no case of GBM with 100% GTR of the tumor. In our study, we consider GTR verified by intraoperative biopsies of the tumor cavity limits and postoperative MRI. Survival time was defined as the time period between diagnosis and patients’ death. In all patients were performed follow-up in scheduled intervals of time. |
| Statistical analysis was performed using IBM SPSS Statistics v12. |
Results
|
| A total of 82 patients were meeting the inclusion criteria for this study, from which 28 female (34.1%) and 54 male (65.9%). The age rage was between 15 and 78 years old, with a mean of 59.56 and a medium of 62 years. KPS of our study group was between 40 and 100. Proliferation associated protein (Ki-67) varied between 10 and 74. From the total of 82 patients, 54 (65.9%) underwent GTR of the tumor and 28 (34.1%) did not. Furthermore, 64 did proceed to combined therapy with RT&TMZ (78%) and 18 did not (22%) (Table 1). |
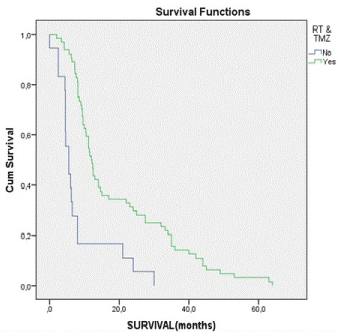
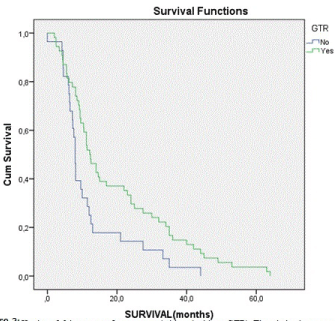
| Concerning the survival of these patients, it varies between 0 and 64 months, with a mean of 16.88 and a median of 10.85 months. In the group of GTR, mean survival reaches 19.44 months, in contrast to the group without GTR, which reaches 11.94 months. Studying the group underwent RT&TMZ, mean survival was about 19.32 months. In those patients, who did nothing but surgery, mean survival was about 8.23 months. Survival curve for patients treated with combination of RT&TMZ, in contrast with that for patients without further treatment after surgery is revelatory (Figure 1). Kaplan-Meier curves are also very informative about the survival of patients with and without GTR (Figure 2). Patients treated with combined therapy RT&TMZ have longer survival, than those with no treatment after surgery. The same proved in the case of patients with and without GTR. In both cases there is a statistically significant correlation (p < 0.05). As far as KPS, Kaplan-Meier curves indicate that patients with moderate KPS (50 to 60) have better outcome (longer survival) and that’s statistical significant, too (p < 0.05). |
| Studying the statistical correlation of individual factors, it is clear that KPS, GTR and RT&TMZ represent statistical significance over survival of the patients. (Tables 2 and 3) All other factors have no statistical significant influence over survival. It seems that GTR promotes the survival. RT&TMZ does the same, too. Studying statistically, combined influence of these factors on survival, it is observed that the correlation is statistically significant (p < 0.04). Correlation between survival and patient management method revealed that patients with combined GTR, RT and TMZ have longer survival time, compared with all others. |
Discussion
|
| Despite new therapies and diagnostic techniques, the median survival interval for patients with GBM still remains very low [6,8,22]. Current standard on GBM treatment is chemo radiotherapy with TMZ following surgical excision [23]. Radiation therapy (RT) has been the main part of the conventional treatment for GBM, until recently, and the chemotherapy has had several limitations [24]. Recently a randomized European and Canadian trial (EORTC 26981/22981-NCIC) has confirmed that the concomitant use of TMZ and RT is a very effective treatment with minimal toxicity in patients with GBM [25]. TMZ is a secondgeneration imidazotetrazine derivative, with cytotoxic influence, because of methylation of specific DNA sites [7]. Cytotoxic effect of TMZ has been considered to be correlated with intracellular levels of O6-methylguanine-DNA-methyltransferase (MGMT) [26]. Moreover, RT and TMZ in newly diagnosed patients with GBM have increased the median survival time, [10,27,28] although in some cases these benefits showed to be significantly compromised [29,30]. Wick et al. reported that for patients with GBM, TMZ therapy with the on/off treatment schedule (1-weekon/ 1-week-off), may be less toxic [30,31]. |
| A large amount of new agents are presently being tested in clinical trials and have demonstrated efficacy [32]. Our consideration of molecular pathways, which are crucial for glioma-genesis and disease development, has been amplified by many studies [33]. Several growth factors such us platelet-derived (PDGF), epidermal (EGF), vascular endothelial (VEGF) and hepatocyte (HGF/SF), are some of the relevant growth factors pathways in gliomas [14]. Specific inhibitors of these targets have been tested successfully in clinical trials and new agents have been developed against these targets, including receptor tyrosine kinases, intracellular signaling molecules, epigenetic abnormalities, tumor vasculature and microenvironment [34]. Immunotherapy has been considered as a hopeful approach in preclinical trials for GBM for more than two decades [35]. For example, there are reports which marked immunohistochemical expression of multidrug resistance protein 5 (MRP5), in surgical tumor specimens of GBM patients, as a prognostic implication [36]. Moreover, another cell surface molecule, CD70, induce potent antitumor immune responses and there is evidence, that a soluble form of CD70 (sCD70) may exhibit biological activity. sCD70 is a potent stimulator for antiglioma immune responses, that depends critically on CD8- positive T cells and could be a very promising adjuvant therapy in future immunotherapy trials against GBM [35]. |
| Despite the new chemotherapeutic strategies, combined multitargeted drugs, cytotoxic chemotherapy and radiotherapy, in order to overcome tumor resistance, no patient with GBM has been cured and the vast majority of patients with glioblastoma experience recurrent disease, with a median time to recurrence of 7 months [36,37]. Only 26.5% of these patients have 2-year survival rate [10,12]. |
| Current standard in GBM therapy includes RT&TMZ, after Safe Maximal Resection (SMR) of the tumor [38], but what about GTR of the tumor? Is there any benefit for the patients’ survival, of the combination of GTR, RT&TMZ? The different rationale of our study is that patients are separated in those who underwent gross total resection and those who did not. That’s mean that in the group of those who did not underwent GTR, included and those who underwent SMR, but not GTR. Moreover, in our study the only available chemotherapy treatment was Temozolomide. The better outcome (longer survival) of the patients suffering GBM, treated with the combination of RT&TMZ has been described [39]. The benefit of GTR has also been proven [40]. Although a good KPS is associating with longer survival, [41] in our study preoperative KPS of about 50-60 is a predictive factor for longer survival. That’s probably, due to the statistical significant correlation between Survival, GTR, KPS and age (p < 0.01). This kind of correlation has been described [42]. Thus, our cohort adding in the combine treatment for GBM, (GTR and RT&TMZ), may provide a more effective therapy eliminating the postoperative disability from this resistant and complex disease. |
| Thus, patients who are allowed by the specificities of the tumor, to undergo a GTR of the tumor, following by RT&TMZ, have predictable longer survival, than those with no GTR and/or no postoperative management with RT&TMZ. |
Conclusion
|
| Generally, the results of clinical studies in GBM therapy are disappointing thus far. Research and development of more promising molecular targeted agents are needed in the laboratory with more synergistic antitumor effects in combination. This study, with the modification of conventional treatment for GBM, adding the TMZ&RT and GTR, under the assistance of microscope, neuro-navigation and Evoked Potentials, could lead to a more efficient management. Before finding treatments and even a cure for this mortal disease, we must achieve better endpoints and should add innovation in order to provide helpful answers. |
Tables at a glance
|
|
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |
| |
| |







