Key words
|
| |
| M. oleifera, hepatoprotective, DMBA, antioxidant properties |
| |
INTRODUCTION
|
| |
| Oxidative stress resulting from the toxic effects of free radicals on the tissue plays an important role in the pathogenesis of various diseases such as Alzheimer’s disease, Parkinson’s disease and Cancer. |
| |
| Free radical induced lipid peroxidation is believed to be one of the major causes of cell membrane damage leading to a number of pathological situations [1]. Epidermiological studies suggest that specific pharmacologically active agents present in the diet might reduce the relative risk of cancer development [2,3]. A remarkable surge of interest in chemoprevention research has, thus, led to the identification of many phytochemicals of dietary origin as effective potential chemopreventive agents [4]. |
| |
| The synthetic polycyclic aromatic hydrocarbon 7,12- dimethylbenz(a)anthracene (DMBA) is a potent carcinogen which is selectively active in sites such as mammary glands, skin, kidney and liver, and has been widely used as a prototype carcinogen in experimental animal models. DMBA exposure induces clinicopathological changes through toxicity occurred to skin, mammary glands, liver and kidney characterized by induction of parenchymal hepatocellular damages including hepatic lesions, tumors and cancer risks [3]. Emerging evidences suggest that DMBA induces the production of reactive oxygen species (ROS) that result in lipid peroxidation, DNA damage, and depletion of cell antioxidant defense systems [4]. Change in lipid peroxidation production reactions and antioxidant defense systems were associated with changes in a variety of biochemical pathways. |
| |
| The liver regulates many important metabolic functions. Hepatic injury is associated with distortion of these metabolic functions [5]. Additionally, it is the key organ of metabolism and excretion, thus it is continuously and variedly exposed to xenobiotics because of its strategic placement in the body. Modern medicines have little to offer for alleviation of hepatic diseases and it is chiefly the plant based preparations that are employed for treatment of liver disorders. But the availability of plant based drugs is scantly for the treatment of liver dysfunction [5,6]. Therefore, many folk remedies from plant origin are evaluated for its possible antioxidant and hepatoprotective effects against different chemical-induced liver carcinogenesis. |
| |
| The traditional Indian medicinal plants act as antiradicals and DNA cleavage protectors [1]. Among myriad of natural plants, Moringa oleifera Lam. (Moringaceae) is called “Miracle vegetable” because of it is both a medicinal and a functional food. Moringa oleifera possess highly therapeutic and pharmacological values, so its consumption in regular diet could possibly reduce the risk of degenerative diseases [3,7]. Hence, the present investigation was designed to explore the influence of M. oleifera hydro-ethanolic pods extract on hepatic oxidative stress marker enzymes in mice with DMBA induced hepato-carcinogenesity. |
| |
MATERIALS AND METHODS
|
| |
|
Chemicals
|
| |
| All chemicals used in the study were of analytical reagent grade and of highest quality available and were purchased from reliable firms (SRL (India), MERCK, RANBAXY, HIMEDIA, QUALLIGENS, Mumbai and SUYOG). DMBA was purchased from SIGMA. Standard kits for LPO, SOD and CAT were obtained from Cayman Chemicals, USA. |
| |
|
Experimental plant
|
| |
| The experimental plant Moringa oleifera was collected from Krishi Vigyan Kendra, Banasthali University, Banasthali, India, in the month of September 2009. The plant material was taxonomically identified by Botanist of Krishi Vigyan Kendra, Banasthali, Tonk district. |
| |
|
Hydro-ethanolic extraction of Plant Material
|
| |
| Dried powdered pods of Moringa oleifera were placed in the Soxhlet thimble with 80% ethanol in 250 ml flat bottom flask. Further refluxed for 18 hours at 800C for two days. Collected solvent were cooled at room temperature and poured in a glass plate. The extract was concentrated under vacuum at 40°C to yield a semisolid mass, dried in hot air oven below 50ºC for 48 hours and stored in a desiccators. The percentage yield of extract was found to be 15.6% and stored at 250C in air tight containers. Suspensions of the extract was prepared in distilled water and used to assess hepatoprotective and antioxidant activity. |
| |
|
Phytochemical Screening
|
| |
| Phytochemical studies of hydroethanolic extract of Moringa oleifera pods showed the presence of alkaloids, flavanoids, steroids, proanthocynadins, tannins, cardiac glycosides and phenols [8,9]. |
| |
|
Total antioxidant capacity
|
| |
| Plant extract at different concentrations (0.1-1 mg/ml)were combined in eppendorf tube with 1ml of reagent solution (0.6M sulphuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). The tubes were capped and incubated in thermal block at 95°C for 90 minutes. After cooling to room temperature; the absorbance of the aqueous solution of each was measured at 695 nm against blank [10]. |
| |
|
Experimental animals
|
| |
| Adult male Swiss albino mice (Mus musculus) weighing 15-30 g were obtained from Haryana Agricultural University, Hissar (India) for experimental purpose. The animals were acclimatized for a month prior to experiment. The Institutional Animal Ethical Committee approved the animal studies. All experiments were conducted on adult male albino mice when they weighed 25-35g (3- 4 months old). Colony bred adult male albino mice were maintained under standard laboratory conditions at a temperature of 22 ± 3ºC, relative humidity of 50±5 % and photoperiod of 12h (12hdark and 12h-light cycle). The mice were housed in polypropylene cages. In order to avoid diurnal variation all the experiments were carried out at same time of the day. Animals lead free access to standard food pellet diet (Hindustan Lever Limited: metal contents in parts per million dry weight: Cu 10.0, Zn 45.0, Mn 55.0, Co 5.0, Fe 75.0) and drinking water ad libitum throughout the study. Essential cleanliness and, to the best extent, sterile condition were also adopted according to SPF facilities. |
| |
|
Acute toxicity studies
|
| |
| Acute oral toxicity was performed as per OECD-423 guidelines [11]. The albino mice were fasted overnight provided only water, after which the hydro-ethanolic extract of the pods of MO was administered by gastric intubation to the relevant animals at the single dose of 15mg/kg body weight. The animals were then observed for 14 days. However, mortality was not observed, the procedure was repeated for further higher doses such as 50, 100, 150, 200, 300, 400, 800, 1600 mg/kg body weight. Mortality was not noticed up to 400 mg/kg, whereas, the LD50 of the extract was found to be 1800 mg/kg body weight. Toxic symptoms for which the animals were observed for 72 h include behavioural changes, locomotion, convulsions and mortality. |
| |
|
Experimental Design
|
| |
| Adult Swiss albino male mice divided into ten groups of 10 mice each group were treated by oral gavage. Treatment consisted of pretreatment phase of MO in distilled water followed by the second phase in which the animals were given 15 mg/kg DMBA on day 15. The animals were then euthanized 4 days after DMBA administration. The groups were as follows- Group 1: served as control (normal untreated mice), and received 1ml distilled water daily by oral gavage. Group 2: received pretreatment with distilled water for 14 days prior to a single dose of DMBA (15 mg/kg body weight: p.o) served as DMBA control group. Group 3 and 4 were administered with hydroethanolic extract of pods of MO (200 and 400 mg/kg body weight: p.o) daily for 14 days, served as MO treated control group. |
| |
| Group 5 and 6: received BHA (0.5 % and 1%: p.o) daily for 14 days, dissolved in 0.5% acetone and served as standard treated control group. |
| |
| Group 7 and 8 were treated with hydro-ethanolic extract of pods of MO (200 and 400 mg/kg body weight; p.o) daily for 14 days, before being intoxicated with DMBA (15 mg/kg body weight; p.o, once) dissolved in olive oil. |
| |
| Group 9 and 10: received BHA (0.5 % and 1%: p.o) daily for 14 days, before being intoxicated with DMBA (15 mg/kg body weight; p.o, once) dissolved in olive oil. |
| |
| The dose for DMBA, standard antioxidant, and plant were decided and selected on the basis of LD50 calculated in the laboratory and on the basis of other published reports [4,12]. |
| |
|
Hepatoprotective activity
|
| |
| After 19 days of duration the mice were fasted overnight and then sacrificed under light ether anesthesia. Liver lobules were dissected out, washed immediately with ice-cold saline to remove blood, and the wet weight noted and then stored at -80°C for various biochemical assays, and histological studies. The enzyme levels were assayed using standard CAYMAN Chemicals assay kits, U.S.A. |
| |
| Preparation of Liver homogenate |
| |
| Liver homogenate was prepared in cold 50 mM potassium phosphate buffer (pH 7.4), for LPO but for SOD and CAT 1mM EDTA was added in it, using Remi homogenizer. The unbroken cells and debris were removed by centrifugation at 10,000 rpm for 15 min at 4°C using a Remi cooling centrifuge and the supernatant was used for the estimation of LPO, SOD, and CAT. |
| |
|
Hepatic oxidative stress Parameters Estimation of lipid peroxidation (LPO)
|
| |
| Cayman’s Lipid hydroperoxide Assay Kit measures the hydroperoxides directly utilizing the redox reactions with ferrous ions [13]. In brief, 0.5 ml of sample was treated with 1 ml of chloroform and centrifuged at 1500g for 5 min. The supernatent was collected and used for assay. The chloroformmethanol solvent (degassed, 2:1, 0.45 ml) was added to 0.5 ml of supernatent followed by addition of 50ul chromagen and incubation for 5 min. Lipid hydroperoxide standards (50 SM ethanolic solution of 13-hydroperoxy octadecadienoic acid) were prepared at different concentrations (0-5 nmol) in chloroform-methanol solvent. The absorbance was measured at 500 nm against reference blank (chloroform-methanol solvent). The LPO was expressed as SM/ gm of protein. |
| |
|
Superoxide dismutase (SOD)
|
| |
| The tissue SOD activity was assayed following the procedure of Cayman’s Superoxide dismutase Kit, which utilizes a tetrazolium salt for detection of superoxide radicals [14]. |
| |
| Liver homogenate (10 Sl) was taken, and 200 Sl of dilute radical detector (24Sm NBT) were added. The reaction was initiated by adding 20 Sl of xanthine oxidase (50 Sl in 1.95 ml 50 mM Tris-HCL, pH 8.0) to all the wells. The control was simultaneously run without liver homogenate. SOD standard (bovine erythocyte SOD) were prepared at different concentrations (0-0.25 U/ml) in 50 mM Tris-HCL, pH 8.0. The plate was incubated for 20 min and the absorbance was measured at 440-460 nm using plate reader. The enzyme activity was expressed as unit ml- 1 and 1 unit of enzyme is defined as amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. |
| |
|
Catalase (CAT)
|
| |
| Catalase activity in the liver was assayed following the procedure of Cayman Catalase Assay Kit, which utilizes the peroxidatic function of CAT for determination of enzyme activity [15,16]. Liver homogenate (20 Sl) was taken with 30 Sl of methanol and 100 Sl of assay buffer (100 mM potassium phosphate, pH 7.0) in two wells. Formaldehyde standards were prepared by addition of 20 Sl formaldehyde (4.25 mM) at different concentrations instead of samples. The reaction was initiated by the addition of 20 Sl of H2O2 (30 mM). Blank without liver homogenate was prepared with 100 Sl of phosphate buffer and 20 Sl of H2O2. The plate was incubated on shaker for 20 min. The reaction was terminated by adding 30 Sl of potassium hydroxide (10 M) to each well and then 30 Sl of purpled (chromogen) was added to each well. This was followed by incubation for 10 min and addition of 10 Sl potassium periodate to each well. The decrease in optical density due to decomposition of H2O2 was measured at the end of 5 min against the blank at 540 nm using plate reader. One unit of activity is equal to the mol of H2O2 degraded min-1 protein at 25º C. The specific activity expressed in terms of units per mg of proteins. |
| |
|
Statistical Analysis:
|
| |
| The experimental results obtained are expressed as mean ± standard deviation (SD) of three replicates. The data was subjected to one way analysis of variance (ANOVA) and differences between samples were determined by Tukey multiple comparison test and Bonferroni’s test using the SPSS 16.0 (Statistical program for Social Sciences) program. The level of significance was set at p<0.001. |
| |
RESULTS
|
| |
|
Total antioxidant capacity (TAC)
|
| |
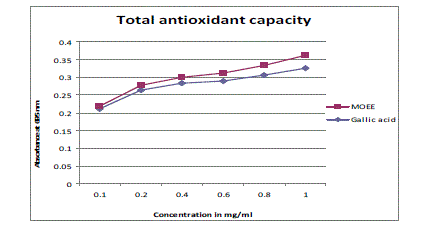
| The TAC exhibited by the plant extract of various concentrations (0.1-1mg/ml) was shown in Figure 1. TAC mainly concentrates on the thermodynamic on the thermodynamic conversion and measures the number of electrons or radicals donated or quenched by a given antioxidant molecule and measure the capacity of biological samples under defined conditions. In this assay extract was found to have higher activity, as compared to the standard used for this study. Thus, the extract-demonstrated electron donating capacity, may act as radical chain terminators, transformating reactive free radical species into stable non-reactive products [17]. |
| |
|
Hepatic oxidative stress Parameters
|
| |
| Findings of the present investigations are summarized in Table 1, which shows the activity of TBARS (thiobarbituric acid reactive substances) and antioxidant related parameters in liver of male mice. Acute toxicity studies indicated that hydro-ethanolic extract of Moringa oleifera pods was found to be non-toxic up to dose of 900mg/ kg body weight. There were no adverse effects of MO on the animals at the given dose levels (200 and 400mg/ kg body weight/day for 14 days respectively). BHA (0.5% and1%) was used as a positive control in the present study. |
| |
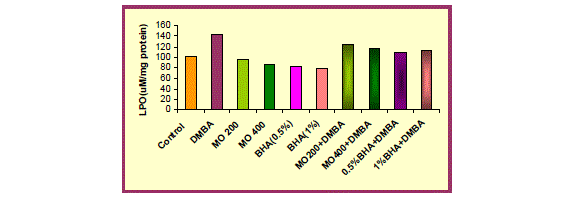
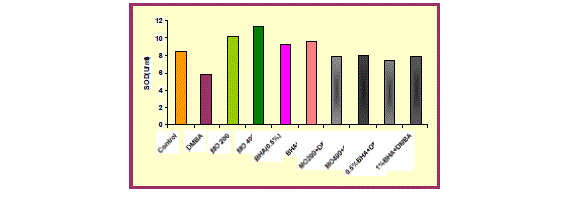
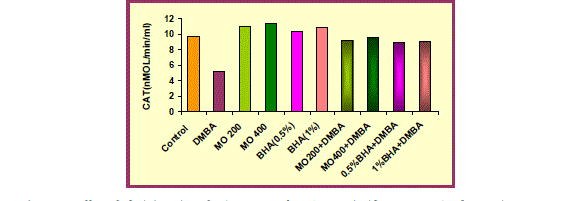
| DMBA (15mg/ kg body weight) exposure was detrimental to the redox status of liver as evidenced by a significant (p<0.001) increase in the Lipid peroxidation level and significant (p<0.001) depletion in CAT and SOD activity, in mice treated with DMBA (group 2) as compared with untreated animals (group 1). This decline in CAT and SOD activity suggest oxidative injury following DMBA exposure. |
| |
| Oral administration of hydro-ethanolic extract of Moringa oleifera (200 and 400mg/ kg body weight) and BHA (0.5% and 1%) significantly (p<0.001 and p<0.01) decreased LPO level but in contrast significant (p<0.001) augmentation was recorded in CAT and SOD activity when compared to control group. |
| |
| In comparison to group 2 (DMBA treated group), group 3 and 4 i.e. MO low dose (200 mg/ kg body weight) and high dose (400mg/ kg body weight) and group 5 and 6 (BHA 0.5%and 1%) significantly (p<0.001) showed a marked decrease in Lipid peroxidation level and a marked increase in CAT and SOD activity, thus protecting liver from oxidative damage due to DMBA. The hepatoprotective effect of the extract was comparable to the effect seen with BHA treatment. |
| |
| Pre-administration of hydro-ethanolic extract of Moringa oleifera at a dose of 200 and 400mg/ kg body weight and BHA (0.5% and 1%) along with DMBA produced a significant (p<0.001) recovery in antioxidant status. An improvement in the LPO level and CAT activity was observed which was accompanied by significant (p<0.001) elevation in SOD activity. |
| |
DISCUSSION
|
| |
| Antioxidants are substances, when present in small quantities prevent the oxidation of cellular organelles by minimizing the damaging effects of ROS and RNS or oxidative stress. All these radicals exert oxidative stress towards the cells of human body and this leads to a number of physiological disorders such as atherosclerosis, arthritis, ischemia, reperfusion injury of many tissues, central nervous system injury, gastritis, cancer and AIDS [18]. Under normal healthy conditions, a balance is maintained between oxidative stress and antioxidant requirements. The endogenous antioxidant defense comes mainly from three different types of systems, viz., antioxidant enzymes e.g. catalase, superoxide dismutase (SOD), metal sequestering proteins e.g. ferritin and low molecular weight molecules like vitamin C, vitamin E etc. However under pathological conditions the balance is lost and excessive supplementation of antioxidants is required. |
| |
| It has been found that fruits and vegetables, rich in antioxidants, decrease the risk of oxidative stress. In this context, the search for new, effective and appropriate antioxidants aimed at minimizing the oxidative stress and providing defense against free radical induced stress in diverse clinical and pathological conditions has gained significant importance. A number of herbal formulations used in traditional Indian medicine are also some of the potent antioxidants, which need to be explored [4]. |
| |
| The present study thus investigates the induction of the activities of hepatic detoxification system enzymes and antioxidant enzyme profiles in mice by Moringa oleifera, Lam drumstick extract. The findings of the present study reveals that administration of the hydro-ethanolic extract of Moringa oleifera at both dose levels (200mg/kg body weight and 400mg/kg body weight) for 14 days daily have enhanced the levels of hepatic superoxide dismutase and catalase, elucidating that Moringa oleifera acts as bifunctional inducer as it induces both Phase-I and Phase-II system enzymes that furnish the balance of xenobiotic metabolism towards detoxification. |
| |
| Active oxygen species and free radicals are involved in a variety of pathological events including cancer. The antioxidant defense enzymes have been suggestive of playing an important role in maintaining physiological levels of oxygen and hydrogen peroxide and eliminating peroxides generated from inadvertent exposure to xenobiotics and drugs. The increase in the levels of antioxidant profiles i.e. SOD and Catalase by Moringa oleifera drumstick extract may be attributed to have biological significance in eliminating reactive free radicals that may affect the normal functioning of cells. |
| |
| In DMBA induced hepatocarcinogenesis in mice the free radical scavenging mechanism pathway may be operating [19] while Dasgupta et al., 2001 [20] has suggested that β-carotene and sterols present in plant acts as potent inhibitors of formation of reactive oxygen intermediates, a pre-requisite for carcinogenesis. The biochemical basis of the chemopreventive potency of Moringa oleifera extract may be attributed to the synergistic action of the constituents of the extract and the induction of antioxidant enzymes, which might be implicated in the anticarcinogenic activity. |
| |
| With reference to antioxidant enzyme status in the liver, almost all the antioxidant-related enzymes, including catalase and superoxide dismutase, were found to be elevated above the control basal values. In the current investigation, induction of SOD activity by MO treatment resulted in inhibition of ROS and dismutation of superoxide radicals into hydrogen peroxide [21]. (Fig 3). |
| |
| Catalase, the activity of which has also been augmented by MO treatment, helped in removing the hydrogen peroxide produced by the action of SOD (Fig. 4). Indeed, SOD activity, along with that of catalase, explains the significant decrease in lipid peroxidation, which is an indicator of oxidative stress that persists in the cell. |
| |
| The present investigation has demonstrated that MO may be used as a cancer chemopreventive agent by virtue of its antioxidant property. The antioxidant property of Moringa may be due to the presence of phenolic compounds that was confirmed in this study by phytochemical screening of the extract [8,9]. In this respect, Moringa pods contain important bioactive compounds including glucosinolates, isothiocyanates, thiocarbamates, and flavonoids [4, 22, 23, 24]. These compounds quench of ROS, chelate metal ions and regenerate membrane-bound antioxidants. This finding is consistent with previous studies, which demonstrated the antioxidant activity of MO extract [8, 9, 25]. |
| |
| MO also protects against oxidative stress via the elevation of antioxidative defense enzyme, while significantly reducing the level of lipid peroxidation [26,27]. All these effects considered together might result in significant reduction of DMBA-induced hepatocarcinogenesis in mice at the peri-initiational period [28]. Since the plants has shown no toxic effect at the tested doses, it could well be applied in cancer chemoprevention, to reduce the risk of cancer. |
| |
CONCLUSION
|
| |
| The results obtained from this study prompt further investigation of the mechanism of hepatoprotective activities of MO and the role of bioactive components of the plant extract responsible for this action. Each part of the plant has been reported useful with various medicinal properties and nutritional values. Therefore, scientifically proven hepatoprotective activities of MO pods may certainly provides benefits following human consumption. |
| |
ACKNOWLEDGMENTS
|
| |
| The authors are grateful to University Grants Commission (UGC) for providing financial assistance. The authors are thankful to the authorities of Banasthali University for providing support to the study. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










