Omkolsoum M Alhaddad1*, Maha M Elsabaawy1, Ahmed Wahb1 and Olfat Hendy2
1 Departments of Hepatology, National Liver Institute, Menoufia University, Shibin El- Kom, Egypt
2 Department of Clinical Pathology, National Liver Institute, Menoufia University, Shibin El-Kom, Egypt
*Corresponding Author:
Omkolsoum M Alhaddad
Department of Hepatology, National Liver Institute
Menoufia University, Shibin El Kom, Egypt.
Tel: 01119388091
E-mail: dromkolsoum@yahoo.com
Received Date: November 07, 2017; Accepted Date: December 01, 2017; Published Date: December 06, 2017
Citation: Alhaddad OM, Elsabaawy MM, Wahb A, Hendy O (2017) Hereditary Spherocytosis Evolving to Non-Hodgkin Lymphoma. Arch Cancer Res. Vol.5 No.4:162. DOI: 10.21767/2254-6081.100162
Keywords
Spherocytosis; Lymphomas; Hematological malignancies; Non-Hodgkin lymphoma
Introduction
Hereditary spherocytosis is the most frequent congenital hemolytic anemia and most common in the Caucasian population [1]. Hereditary spherocytosis is a familial disorder with both autosomal dominant and recessive modes of inheritance, but sporadic cases can be encountered [2,3]. The clinical presentation is widely arrayed from a severe course in early childhood to asymptomatic and milder forms in elderly. The hallmarks of hereditary spherocytosis are the chronic hemolytic sequelae in presence of spherocytes at peripheral blood smear and positivity of red cell fragility testing [4-7].
Case Presentation
54-year-old lady had presented to the emergency room of National Liver Institute Hospital, Menoufia University by marked shortness of breath, dizziness and icterus. The patient was leading a completely healthy life till three years ago when she was hospitalized for a similar attack of dyspnea and jaundice. She received 5 units or more of blood and was discharged in a fair condition without any further work up. Six months earlier; she started to suffer anorexia, significant weight loss along with severe bony pains all over the body, but no fever, abdominal pain, lymph node swellings or bleeding. The clinical examination revealed a cachectic woman with bounding pulse, jaundice and huge splenomegaly crossing the midline. Laboratory investigations revealed marked anemia with elevated MCV and MCHC (132% and 45%) though, serum vitamin B12 and folic acid were normal (Table 1). The evident indirect hyperbilirubinemia, significant reticulocytosis, and elevated serum LDH were associated with negative direct and indirect Coomb's tests. Abdominal sonography demonstrated huge splenomegaly (29 cm) with homogenous echo-pattern along with calcular gall bladder. Furthermore, red cell distribution width (RDW) was found to be surmounted to 17% in favor of the diagnosis of HS. Osmotic fragility test was increased (showed early RBCs hemolysis at low dilution titers). Although a definite family history was lacking, the diagnosis of hereditary spherocytosis (HS) was ascertained. Four units of packed RBCs were advised; however, rapid regression of hemoglobin had occurred within 2 days.
| Variables |
On Admission |
| Total bilirubin (mg/dl) |
8.7 |
| Direct bilirubin (mg/dl) |
2.5 |
| Albumin (gm/dl) |
4.1 |
| AST (IU/dl) |
169 |
| ALT (IU/dl) |
188 |
| LDH (IU/dl) |
3211 |
| HG (gm/dl) |
5 |
| Retics (103/mm) |
11 |
| MCV (µm3) |
110 |
| MCHC (gm/dl) |
37 |
| WBCs (103/mm) |
18 |
| Platelets (103/mm) |
115 |
Table 1: Laboratory investigation data of patient.
The high ESR: 120 mm/1st hr and B2 macroglobulin: 7.5 ug/ml added to the significant weight loss had prompted referring the patient to bone marrow studies.
Bone marrow (BM) trephine biopsy unveiled the presence of Non-Hodgkin lymphoma (Figures 1 and 2) which identified by aggregates of typical mature hematopoietic tissue, some mature megakaryocytes and an increase of erythroid elements (Erythroid Hyperplasia). Furthermore, the trephine showed a small number of mature myeloid cells but dysplastic changes or increasing blast cells were not detected. Other sites of the trephine showed diffuse infiltration by lymphoid tissue population of small mature cells which having indented hyperchromatic nuclei and some large single nucleolus suggesting the diagnosis of diffuse follicular Non-Hodgkin lymphoma. Such diagnosis was confirmed by immunohistochemical staining that showed a positivity for B-cell markers, CD10, CD19, CD22, CD79a and strongly positive for CD20 and Bcl2 (Figures 3 and 4) and negative for CD5, CD30 and CD138. Meanwhile, PET-CT scan of the whole body showed increased FDG uptake in the spleen and bone marrow. The patient was referred to the oncology unit; unfortunately, she passed before access to proper therapeutic interventions.
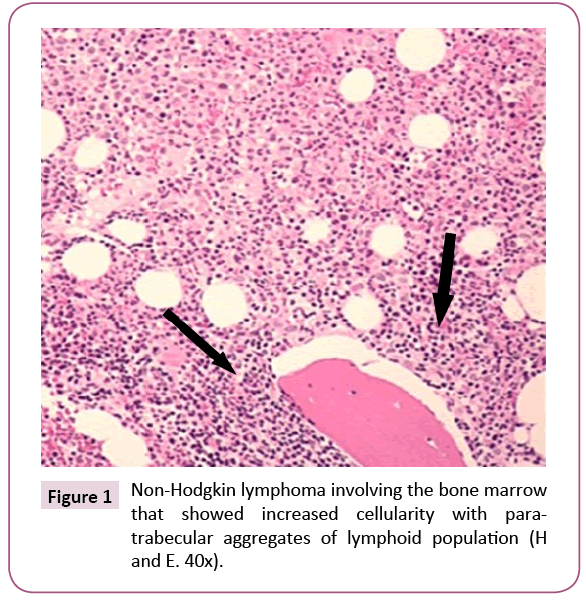
Figure 1: Non-Hodgkin lymphoma involving the bone marrow that showed increased cellularity with paratrabecular aggregates of lymphoid population (H and E. 40x).
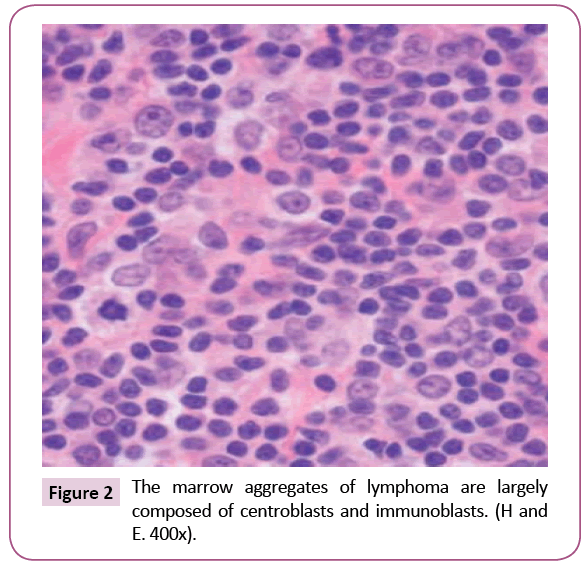
Figure 2: The marrow aggregates of lymphoma are largely composed of centroblasts and immunoblasts. (H and E. 400x).
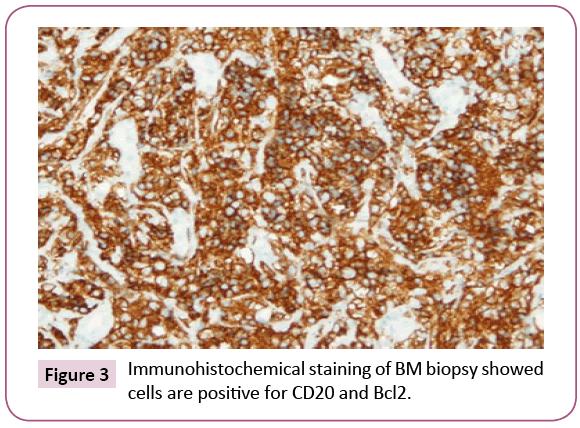
Figure 3: Immunohistochemical staining of BM biopsy showed cells are positive for CD20 and Bcl2.
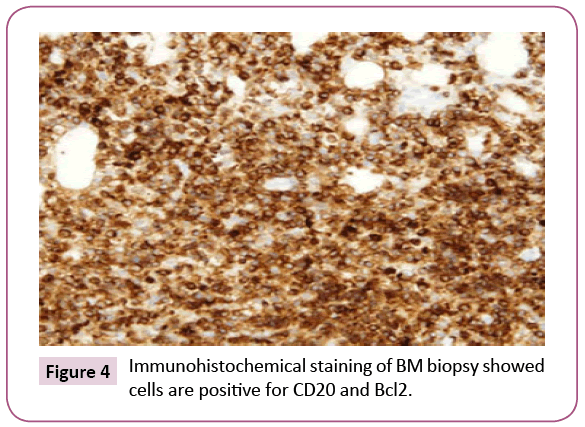
Figure 4: Immunohistochemical staining of BM biopsy showed cells are positive for CD20 and Bcl2.
Conclusion and Learning Points
1. Hereditary spherocytosis is an inherited form of chronic hemolytic anemia; it occurs secondary to the cytoskeletal proteins deficit in the erythrocyte membrane (Band 3, Ankyrin 1, Proteins 4.2, Alpha or Beta Spectrin).
2. Milder forms of HS often escape clinical recognition and the diagnosis may be an incidental laboratory finding.
3. Also, the diagnosis can be prevailed with the increased hemolysis that may accompany viral illnesses, probably due to splenomegaly associated with the viral illness.
4. HS has been rarely described in association with lymphomas and hematological neoplasms.
5. Chronic spherocytic hemolysis and long-standing stimulation of both bone marrow and spleen may represent a pathogenic background of hyperactivation of B cells and lymphomatous transformation.
Conflict of Interest
The authors declare that they have no conflict of interest that competes with any of the contents of the manuscript.
21345
References
- Tse WT, Lux SE (1999) Red blood cell membrane disorders. Br J Haematol104:2-13.
- Bianchi P, Fermo E, Vercellati C, Marcello AP, Porretti L, et al. (2012) Diagnostic power of laboratory tests for hereditary spherocytosis: A comparison study in 150 patients grouped according to molecular and clinical characteristics. Haematologica 97: 516-523.
- Bolton-Maggs PH, Stevens RF, Dodd NJ, Lamont G, Tittensor P, et al. (2004) General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis and management of hereditary spherocytosis. Br J Haematol.126:455-74.
- Arisawa K, Morita S, Kojima H, Inui A, Yoshino G,et al. (1994) Hereditary spherocytosis associated with non-Hodgkin's lymphoma in the spleen. RinshoKetsueki 35:871-875.
- Koch CA, Li CY, Mesa RA, Tefferi A (2003) Non-hepatosplenic extramedullary hematopoiesis: Associated diseases, pathology, clinical course and treatment. Mayo ClinProc78:1223-1233.
- Molina-Urra R, Martinez D, Sagasta A, Carrio A, Setoain X, et al. (2015) Paraspinal extramedullary hematopoiesis in hereditary spherocytosis with a concurrent follicular lymphoma: Case report and review of the literature. DiagnPathol 10: 158.
- OMalley DP (2007) Benign extramedullary myeloid proliferations. Mod Pathol20:405-415.









